These are some PYQs (previous year questions) from the CBSE board for Class 10 Science, Chapter 1: Chemical Reactions and Equations.
By practicing these questions, you will get an idea of what type of questions may appear in the examination.
Question 1:
The emission of brown fumes in the given experimental set-up is due to…
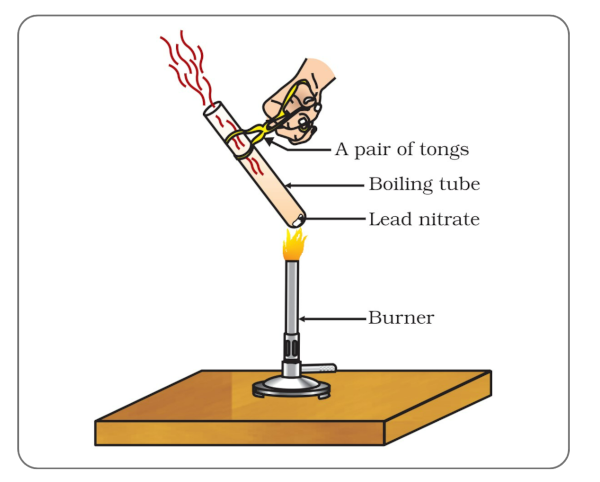
(a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
(b) thermal decomposition of lead nitrate which produces brown fumes of lead oxide.
(c) oxidation of lead nitrate forming lead oxide and nitrogen dioxide.
(d) oxidation of lead nitrate forming lead oxide and oxygen.
1 mark; CBSE 2022
Answer:
(a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
Question 2:
Write the essential condition for the following reaction to take place:
2AgBr → 2Ag + Br2
Write application of this reaction.
2 marks; CBSE 2016
Answer:
The reaction will take place in presence of sunlight.
This reaction is used in black and white photography.
Question 3:
Name the reducing agent in the following reaction:
3MnO2 + 4Al → 3Mn + 2Al2O3
State which is more reactive, Mn or Al and Why?
2 marks; CBSE 2016, 2015
Answer:
Al is the reducing agent in the given reaction.
Al is more reactive than Mn.
Reason: It is because Al is displacing Mn from MnO2.
Question 4:
What is observed when a solution of potassium iodide is added to a solution of lead nitrate? Name the type of reaction. Write a balanced chemical equation to represent the above chemical reaction.
2 marks; CBSE 2014, 2013
Answer:
Yellow colored solid precipitate is formed due to formation of lead iodide.
It is a precipitation reaction as well as double displacement reaction.
The balanced chemical equation is shown below;
Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
Question 5:
Using balanced chemical equation explain the difference between a displacement reaction and a double displacement reaction.
2 marks; CBSE 2012, 2011
Answer:
Displacement reaction: A reaction in which a more reactive element displaces a less reactive element from its salt solution.
For example;
Fe + CuSO4 → FeSO4 + Cu
Double displacement reaction: A reaction in which two compounds exchange their ions to form two new compounds.
For example;
NaOH + HCl → NaCl + H2O
Question 6:
When a solution of potassium iodide is added to a solution of lead nitrate in a test tube, a reaction takes place.
a. What type of reaction is this?
b. Write the balanced chemical equation to represent the above reaction.
2 marks; CBSE 2011, 2010, 2008
Answer:
a. The reaction is a precipitation reaction as well as double displacement reaction.
b. The balanced chemical equation is shown below;
Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
Question 7:
What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride? State the physical conditions of reactants in which the reaction between them will not take place. Write the balanced chemical equation for the reaction and name the type of reaction.
2 marks; CBSE 2016, 2010
Answer:
White precipitate of BaSO4 is formed.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
It is a double displacement reaction.
If reactants are taken in solid state, products will not be formed.
Question 8:
What is redox reaction? When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidized or reduced. Why?
2 marks; CBSE 2010, 2009
Answer:
Redox reaction is a reaction in which oxidation and reduction takes place simultaneously.
Mg is getting oxidized because it is gaining oxygen to form magnesium oxide (MgO).
Question 9:
Why do we store silver chloride in dark coloured bottle? Explain in brief.
2 marks; CBSE 2010
Answer:
Silver chloride is stored in dark bottles in order to cut off the exposure to sunlight. AgCl is photosensitive, it will decompose to Ag and Cl2 in the presence of sunlight.
2AgCl(s) + sunlight → 2Ag(s) + Cl2(g)
Question 10:
In the electrolysis of water:
a. Name the gas collected at the cathode and anode respectively.
b. Why is volume of gas collected at one electrode double than that at the other? Name this gas.
c. How will you test this gas?
3 marks; CBSE 2012, CBSE Sample Paper 2018
Answer:
a. Hydrogen gas is collected at the cathode and oxygen gas is collected at the anode.
b. It is because H2O contains hydrogen and oxygen in the ratio 2:1.
c. Testing of gas can be done by bringing a burning matchstick near the gas. If the gas burns with ‘pop’ sound, the gas is H2.
Question 11:
(i) Solid calcium oxide was taken in a container and water was added slowly to it.
(a) Write the observations.
(b) Write the chemical formula of the product formed.
(ii) What happens when carbon dioxide is bubbled through lime water
(a) in small amount
(b) in excess?
3 marks; CBSE 2013, 2012, 2010
(i)
(a) The container becomes hot and hissing sound is produced.
(b) The formula of the product formed is Ca(OH)2.
(ii)
(a) Lime water turn milky when CO2 gas is passed through it:
Ca(OH)2 + CO2 → CaCO3 + H2O
(b) If excess of CO2 is passed, the milkiness disappears:
CaCO3 + H2O + CO2 → Ca(HCO3)2
Question 12:
(a) Why is it necessary to balance a chemical equation?
(b) Write the balanced chemical equation for the following reactions:
(i) Natural gas burns in air to form carbon dioxide and water.
(ii) During respiration, glucose combines with oxygen and forms carbon dioxide and water along with the release of energy.
3 marks; CBSE 2013
Answer:
a. Chemical equation must be balanced so as to follow the law of conservation of mass.
b.
(i) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
(ii) C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) + Heat
Question 13:
Identify the type of reactions taking place in each of the following:
a. Barium chloride solution is mixed with copper sulfate solution and white precipitate is formed.
b. On heating copper powder in china dish, the surface of copper powder turns black.
c. On heating green coloured ferrous sulfate crystals, reddish brown solid is left and smell of a gas having odor of burning sulfur is experienced.
d. Iron nails when left dipped in blue copper sulfate solution become reddish brown in colour and the blue colour of copper sulfate fades away.
e. Quick lime reacts vigorously with water releasing a large amount of heat.
5 marks; CBSE Sample Paper 2009
Answer:
a. Double displacement reaction,
b. Oxidation,
c. Decomposition reaction,
d. Displacement reaction,
e. Combination reaction.
Question 14:
Identify the type of chemical reaction in the following statement and define each of them:
a. Digestion of food in our body.
b. Rusting of iron.
c. Heating of manganese dioxide with aluminum powder.
d. Blue colour of copper sulfate solution disappears when iron filings are added to it.
e. Dilute hydrochloric acid is added to sodium hydroxide solution to form sodium chloride and water.
5 marks; CBSE 2016
Answer:
a. Decomposition reaction: It is a process in which a compound is broken down into simple substances.
b. Oxidation: The process in which oxygen is added or electrons are lost.
c. Displacement reaction: The reaction in which a more reactive element can displace a less reactive element from its salt solution.
d. Displacement reaction: The reaction in which a more reactive element can displace a less reactive element.
e. Neutralization reaction: The reaction in which acid reacts with base to form salt and water.
