These are some PYQs (previous year questions) from the CBSE board for Class 10 Science, Chapter 4: Carbon and its Compounds.
By practicing these questions, you will get an idea of what type of questions may appear in the examination.
Question 1:
Carbon compounds:
(i) are good conductors of electricity.
(ii) are bad conductors of electricity.
(iii) have strong forces of attraction between their molecules.
(iv) have weak forces of attraction between their molecules.
The correct statements are:
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iii)
1 mark; CBSE 2024
Answer:
(c)
Question 2:
Consider the following statements about homologous series of carbon compounds :
(A) All succeeding members differ by — CH2 unit.
(B) Melting point and boiling point increases with increasing molecular mass.
(C) The difference in molecular masses between two successive members is 16 u.
(D) C2H2 and C3H4 are NOT the successive members of alkyne series.
The correct statements are:
(a) (A) and (B)
(b) (B) and (C)
(c) (A) and (C)
(d) (C) and (D)
1 mark; CBSE 2024
Answer:
(a)
Question 3:
Assertion (A): Ethanoic acid is also known as glacial acetic acid.
Reason (R): The melting point of pure ethanoic acid is 290 K and hence it often freezes during winters
in cold climates.
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
1 mark; CBSE 2020
Answer:
(a)
Pure ethanoic acid or acetic acid freezes below room temperature into white crystals that resemble
glaciers.
Question 4:
Several factories were pouring their wastes in rivers A and B. Water samples were collected from these two rivers. It was observed that sample collected from river A was acidic while that of river B was basic. The factories located near A and B are:
(a) Soaps and detergents factories near A and alcohol distillery near B.
(b) Soaps and detergents factories near B and alcohol distillery near A.
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
(d) Lead storage battery manufacturing factories near B and soaps and detergents factories near A.
1 mark; CBSE 2020
Answer:
(c)
Question 5:
Explain why cannot we have isomers of first three members of alkane family.
2 marks; CBSE Sample Paper 2017, CBSE 2015
Answer:
Branching is not possible with carbon atoms until propane, which is why there are no isomers before it.
Question 6:
Why are detergents preferred over soaps for washing clothes in hard water? Explain.
2 marks; CBSE 2015, 2014
Answer:
Detergents work effectively with hard water because their calcium and magnesium salts dissolve easily, preventing scum formation.
Question 7:
How do the melting and boiling points of the hydrocarbons change with increase in molecular mass?
2 marks; CBSE 2012
Answer:
The melting and boiling points of hydrocarbons go up as their molecular mass increases. This is because a larger molecular mass means a bigger surface area, which leads to stronger van der Waals forces between the molecules.
Question 8:
Carbon does not form ionic compounds, why?
2 marks; CBSE 2015, 2013
Answer:
Carbon can’t lose four electrons because it requires a lot of energy to do so. Similarly, it can’t gain four electrons because its six protons can’t hold ten electrons. That’s why carbon doesn’t form ionic compounds.
Question 9:
Give reasons for the following:
a. Unsaturated hydrocarbons show addition reactions but not saturated hydrocarbons.
b. Carbon forms only covalent bonds.
2 marks; CBSE 2011
Answer:
a. Unsaturated hydrocarbons have double or triple bonds, which allow more molecules to be added. In contrast, saturated hydrocarbons have only single bonds, so they don’t react in this way.
b. It can’t lose four electrons because that would require too much energy, and it can’t gain four electrons because six protons can’t hold ten electrons. Instead, it shares four electrons to form covalent bonds and become stable.
Question 10:
An aldehyde as well as ketone can be represented by the same molecular formula say C3H6O. Write their structures and name them. State the relationship between two in language of science.
3 marks; [CBSE Sample Paper 2017-2018, CBSE 2016]
Answer:
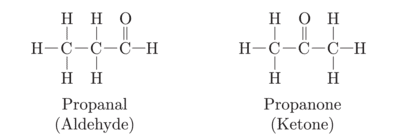
These are functional isomers.
Question 11:
Explain giving reasons, why carbon can neither form C4+ cation nor C4- anion but forms covalent compounds which are bad conductors of electricity and have low melting and boiling points.
3 marks; CBSE 2017
Answer:
Carbon cannot lose four electrons because it requires too much energy to remove them. It also cannot gain four electrons because its six protons cannot hold ten electrons. Instead, carbon shares four electrons to form covalent bonds.
Covalent compounds do not conduct electricity because they don’t form ions. They have low melting and boiling points because the forces holding their molecules together are weak.
Question 12:
Write the molecular formula of benzene and draw its structure. List in tabular form how covalent compounds differ from ionic compounds.
3 marks; CBSE 2017
Answer:
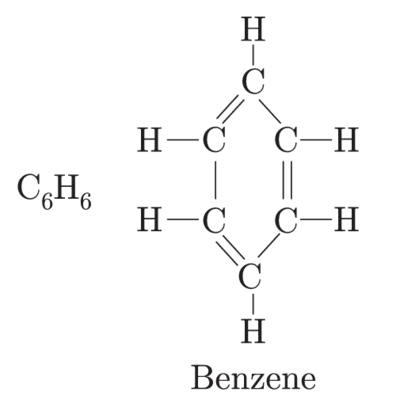
The differences between covalent compounds and ionic compounds are listed below:
| Covalent compounds | Ionic compounds |
| They are bad conductor of electricity. | They are good conductor of electricity. |
| They have low melting and boiling points. | They have high melting and boiling points. |
Question 13:
Write any three physical properties and three uses of ethanol.
3 marks; CBSE 2016
Answer:
Properties:
- Ethanol has a distinct smell.
- It dissolves in water.
- It has a burning taste.
Uses:
- It is used to make ethanoic acid and ethyl ethanoate (esters).
- It is used as a solvent.
- It acts as an antiseptic.
- It is found in beverages like wine, beer, and whisky.
Question 14:
Give reasons for the following:
a. Element carbon forms compound mainly by covalent bonding.
b. Diamond has high melting point.
c. Graphite is good conductor of electricity.
d. Acetylene bums with sooty flame. e. Kerosene does not decolourise bromine water whereas cooking oil does.
5 marks; CBSE 2011
Answer:
Carbon can’t lose or gain four electrons, so it shares them to form covalent bonds.
– Kerosene is a saturated compound, so it doesn’t change the color of bromine water.
– Diamond has strong C—C bonds and a compact 3-D structure where each carbon atom is bonded to four other carbon atoms, giving it a high melting point.
– Graphite has each carbon atom bonded to three other carbon atoms, leaving one free electron per carbon that allows it to conduct electricity.
– Acetylene has a lot of carbon, so when it’s partially oxidized, it burns with a sooty or smoky flame.
