These are some PYQs (previous year questions) from the CBSE board for Class 10 Science; Chapter 2: Acids, Bases and Salts.
By practicing these questions, you will get an idea of what type of questions may appear in the examination.
Question 1:
The change in color of the moist litmus paper in the given set up is due to…
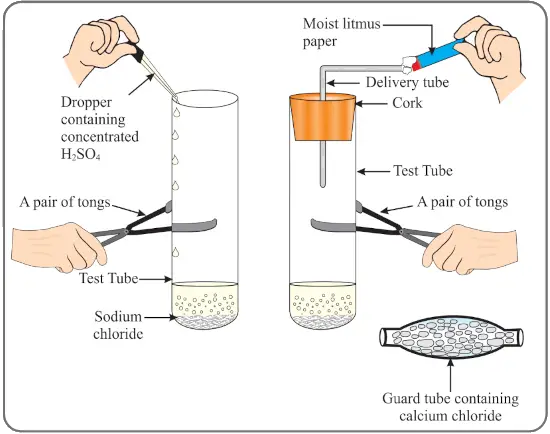
(i) Presence of acid.
(ii) Presence of base.
(iii) Presence of H⁺(aq) in the solution.
(iv) Presence of Litmus which acts as an indicator.
(a) i and ii
(b) only ii
(c) only iii
(d) only iv
1 mark; CBSE 2022
Question 2:
Three acidic solutions A, B and C have pH = 0, 3 and 5 respectively.
a. Which solution has the highest concentration of H+ ions?
b. Which solution has the lowest concentration of H+ ions?
1 mark; CBSE 2015
Answer:
a. The solution with pH = 0 has highest concentration of H+ ions.
b. The solution with pH = 5 has lowest concentration of H+ ions.
Question 3:
A white chemical compound becomes hard on mixing proper quantity of water. It is also used to maintain joints in fixed position. Name the chemical compound and write its chemical formula. Write the chemical equation to show what happens when water is added to this compound in proper quantity.
2 marks; CBSE 2016
Answer:
CaSO4 . 1/2 H2O is the formula of the compound. The name of compound is ‘Plaster of Paris’ (Calcium sulphate hemihydrate).
And the chemical equation for reaction of POP with water is as below:
CaSO4 . 1/2 H2O + 3/2 H2O → CaSO4 . 2H2O
Question 4:
Two solutions ‘A’ and ‘B’ have pH value 3.0 and 10.5 respectively. Which of these will turn
a. Blue litmus solution to red,
b. Phenolphthalein from colourless to pink?
Justify your answer in each case.
2 marks; CBSE 2016
Answer:
a. ‘A’ with pH = 3, will turn blue litmus red because it is acidic in nature.
b. ‘B’ with pH = 10.5, will turn phenolphthalein colourless to pink because ‘B’ is basic in nature.
Question 5:
What is chlor-alkali process? Write a balanced chemical equation for the reaction involved in this process, to justify your answer.
2 marks; CBSE 2016
Answer:
When brine solution is electrolysed we get alkali (NaOH) and chlorine (Cl2) gas, this process is called chlor-alkali process.
The chemical reaction for this reaction is as follows:
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Question 6:
15 mL of water and 10 mL of sulphuric acid are to be mixed in a beaker
a. State the method that should be followed with reason.
b. What is this process called?
2 marks; CBSE 2015
Answer:
a. Acid should be added to the water slowly with constant cooling because the reaction is highly exothermic.
b. This process is called dilution.
Question 7:
Name the acid present in the following:
a. Tomato,
b. Vinegar,
c. Tamarind
2 marks; CBSE 2015
Answer:
a. Tomato contains oxalic acid.
b. Vinegar contains acetic acid.
c. Tamarind contains tartaric acid.
Question 8:
What is the action of litmus on
a. dry ammonia gas
b. solution of ammonia gas in water?
2 marks; CBSE 2016
Answer:
a. There is no effect of dry litmus on dry ammonia gas.
b. Solution of ammonia will turn red litmus blue.
Question 9:
A student detected the pH of four unknown solutions A, B, C and D as follows: 11, 5, 7 and 2. Predict the nature of these solutions.
2 marks; CBSE 2013
Answer:
pH = 11 is basic
pH = 5 is acidic
pH = 7 is neutral
pH = 2 is strongly acidic
Question 10:
Give two uses of baking soda and washing soda each.
2 marks; CBSE 2013
Answer:
Use of baking soda:
a. It is used in making of bread, biscuits, cakes.
b. It is used as an antacid.
Use of washing soda:
a. It is used as a cleansing agent.
b. It is used to remove hardness of water.
Question 11:
A compound ‘X’ of sodium is commonly used for making crispy pakoras. It is also used for curing acidity in the stomach. Identify ‘X’. Write the formula and its chemical name. State the reaction which takes place when it is heated.
2 marks; CBSE 2013, 2008
Answer:
The compound ‘X’ is NaHCO3 , sodium hydrogen carbonate. It is used in cooking and for curing acidity in stomach.
The chemical reaction is as mentioned below;
2NaHCO3 + heat → Na2CO3 + CO2 + H2O
Question 12:
(a) Write the name given to the bases that are highly soluble in water. Give an example.
(b) Why does bee sting causes pain and irritation? Rubbing of baking soda on the sting area gives relief. How?
2 marks; CBSE 2012
Answer:
(a) Highly soluble bases are called alkalies, for e.g., KOH.
(b) Bee sting contains HCOOH, formic acid which causes irritation. Baking soda (basic) neutralises HCOOH, therefore it gives relief from pain on rubbing it on sting area.
Question 13:
The pH of the mouth of a person is lower than 5.5. What changes will occur in his mouth? How these changes can be controlled? Write any two measures.
2 marks; CBSE 2012, 2011, 2010
Answer:
Acid will be formed in the mouth which causes tooth decay.
a. Wash your mouth with water after every meal.
b. Brush your teeth after meal.
Toothpastes are basic in nature and it will neutralise the acid formed in mouth.
Question 14:
Answer the following questions:
a. State the colour of phenolphthalein in soap solution.
b. Name the by-product of chlor-alkali process which is used for the manufacture of bleaching powder.
c. Name one indicator which specifies the various levels of H+ ion concentration.
3 marks; CBSE 2016
Answer:
a. Phenolphthalein will turn pink in soap solution.
b. Chlorine is the by-product of chlor-alkali process which is used in the manufacture of bleaching powder.
c. Universal indicator specifies the various levels of H+ ion concentration.
Question 15:
2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation for the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid.
3 marks; CBSE 2018
Answer:
Zn(s) + 2NaOH(aq) → Na2ZnO2(s) + H2(g)
This gas can be tested by bring a burning candle near the gas. If it burns with ‘pop’ sound, the gas liberated is hydrogen gas.
Zn(s) + H2SO4(dil) → ZnSO4(aq) + H2(g)
Hydrogen gas will be evolved by reaction of the same metal with dilute H2SO4 (strong acid).
Question 15:
a. Identify the acid and the base whose combination forms the common salt that you use in your food. Write its chemical formula and chemical name of the salt.
b. What is rock salt? Mention its colour and the reason due to which it has this colour.
c. What happens when electricity is passed through brine? Write chemical equation for it.
5 marks; CBSE 2013
Answer:
a. NaOH (Sodium hydroxide) and HCl (Hydrochloric acid) form common salt. NaCl is common salt, sodium chloride.
b. Rock salt is sodium chloride found in the form of rocks. It is yellowish in colour due to the presence of impurities.
c. Sodium hydroxide, H2 gas and chlorine gas will be formed:
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + H2(g) + Cl2(g)