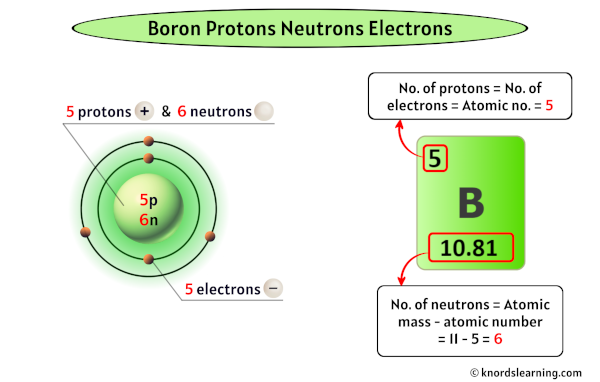
Boron has 5 protons, 6 neutrons and 5 electrons.
But how will you find the number of protons, neutrons and electrons in Boron (B)?
Well, it is very easy to find the protons, neutrons and electrons of boron atom.
Here I have given a very simple method for finding the protons, neutrons and electrons of boron atom.
Let’s dive right into it!
Finding the Protons, Neutrons and Electrons in Boron
How to find protons?
- The number of protons can be found by knowing the atomic number of that atom. [1]
How to find neutrons?
- The number of neutrons can be found by subtracting the atomic number from its atomic mass.
How to find electrons?
- For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.
Let’s calculate the number of protons, neutrons and electrons in boron.
If you don’t want to read, then you can also watch this video.
#1 Number of Protons in Boron
If you have a periodic table with you, then most of the answers are in front of you.

You can see the elements, their atomic number and their atomic mass from the periodic table.
Now here our element is Boron (B).

So from the above periodic table, you can see that the atomic number of boron is 5.
As the atomic number of boron is 5, it has a total of 5 protons in its nucleus.
Thus, the number of protons in Boron is 5.
#2 Number of Neutrons in Boron
In order to find the number of neutrons of boron atom, you should know its atomic mass first.
The number of neutrons in boron can be obtained by subtracting the atomic number from its atomic mass.
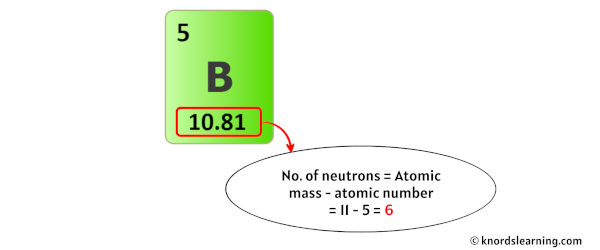
The atomic mass of boron is 10.81 u (which you can round it to 11). [2]
So from this atomic mass (i.e 11), you have to subtract its atomic number (i.e 5).
So you will get 11 – 5 = 5.
Thus, the number of neutrons in Boron is 6.
#3 Number of Electrons in Boron

For a neutral atom, the number of electrons and the number of protons are the same.
Here, the boron atom is a neutral atom.
So the number of electrons in boron is equal to its number of protons (which is also equal to its atomic number).
In the beginning, we have found that the number of protons in boron is 5.
Thus, the number of electrons in Boron is 5.
Summary
Number of Protons in Boron
- The number of protons can be found by knowing the atomic number of that atom.
- Number of Protons in Boron = Atomic number of Boron = 5
Number of Neutrons in Boron
- The number of neutrons can be found by subtracting the atomic number from its atomic mass.
- Number of Neutrons in Boron = Atomic mass of Boron – Atomic number of Boron = 11 – 5 = 6
Number of Electrons in Boron
- For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.
- Number of Electrons in Boron = Atomic number of Boron = 5
I hope you have understood the simple method for finding the protons, neutrons and electrons of boron atom.
Check out related topics for more practice;
Carbon protons neutrons electrons
Nitrogen protons neutrons electrons
Oxygen protons neutrons electrons
Fluorine protons neutrons electrons
Neon protons neutrons electrons
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.