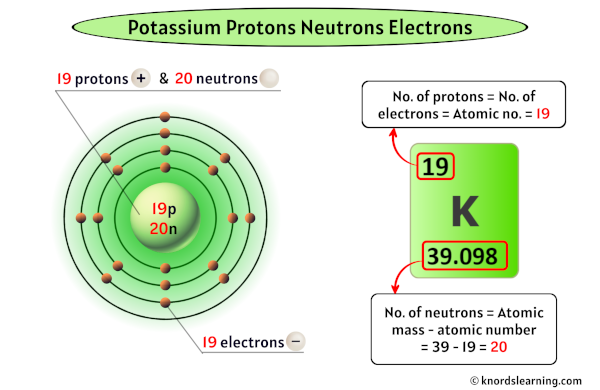
Potassium has 19 protons, 20 neutrons and 19 electrons.
But how will you find the number of protons, neutrons and electrons in Potassium (K)?
Well, it is very easy to find the protons, neutrons and electrons of potassium atom.
Here I have given a very simple method for finding the protons, neutrons and electrons of potassium atom.
Let’s dive right into it!
Finding the Protons, Neutrons and Electrons in Potassium
How to find protons?
- The number of protons can be found by knowing the atomic number of that atom. [1]
How to find neutrons?
- The number of neutrons can be found by subtracting the atomic number from its atomic mass.
How to find electrons?
- For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.
Let’s calculate the number of protons, neutrons and electrons in potassium.
If you don’t want to read, then you can also watch this video.
#1 Number of Protons in Potassium
If you have a periodic table with you, then most of the answers are in front of you.

You can see the elements, their atomic number and their atomic mass from the periodic table.
Now here our element is Potassium (K).
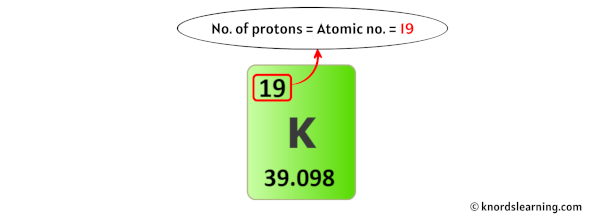
So from the above periodic table, you can see that the atomic number of potassium is 19.
As the atomic number of potassium is 19, it has a total of 19 protons in its nucleus.
Thus, the number of protons in Potassium is 19.
#2 Number of Neutrons in Potassium
In order to find the number of neutrons of potassium atom, you should know its atomic mass first.
The number of neutrons in potassium can be obtained by subtracting the atomic number from its atomic mass.
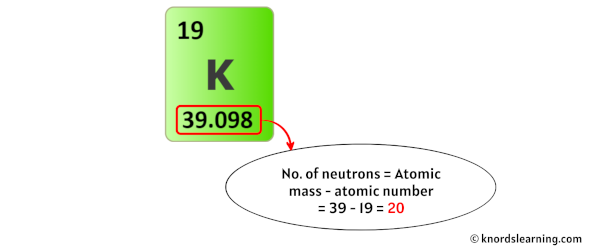
The atomic mass of potassium is 39.098 u (which you can round it to 39). [2]
So from this atomic mass (i.e 39), you have to subtract its atomic number (i.e 19).
So you will get 39 – 19 = 20.
Thus, the number of neutrons in Potassium is 20.
#3 Number of Electrons in Potassium
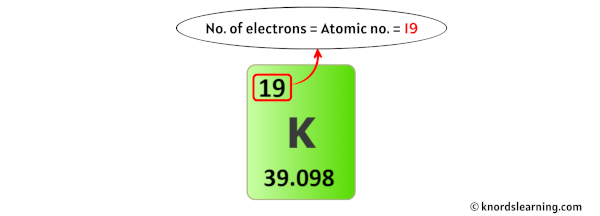
For a neutral atom, the number of electrons and the number of protons are the same.
Here, the potassium atom is a neutral atom.
So the number of electrons in potassium is equal to its number of protons (which is also equal to its atomic number).
In the beginning, we have found that the number of protons in potassium is 19.
Thus, the number of electrons in Potassium is 19.
Summary
Number of Protons in Potassium
- The number of protons can be found by knowing the atomic number of that atom.
- Number of Protons in Potassium = Atomic number of Potassium = 19
Number of Neutrons in Potassium
- The number of neutrons can be found by subtracting the atomic number from its atomic mass.
- Number of Neutrons in Potassium = Atomic mass of Potassium – Atomic number of Potassium = 39 – 19 = 20
Number of Electrons in Potassium
- For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.
- Number of Electrons in Potassium = Atomic number of Potassium = 19
I hope you have understood the simple method for finding the protons, neutrons and electrons of potassium atom.
Check out related topics for more practice;
Calcium protons neutrons electrons
Strontium protons neutrons electrons
Barium protons neutrons electrons
Francium protons neutrons electrons
Boron protons neutrons electrons
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
