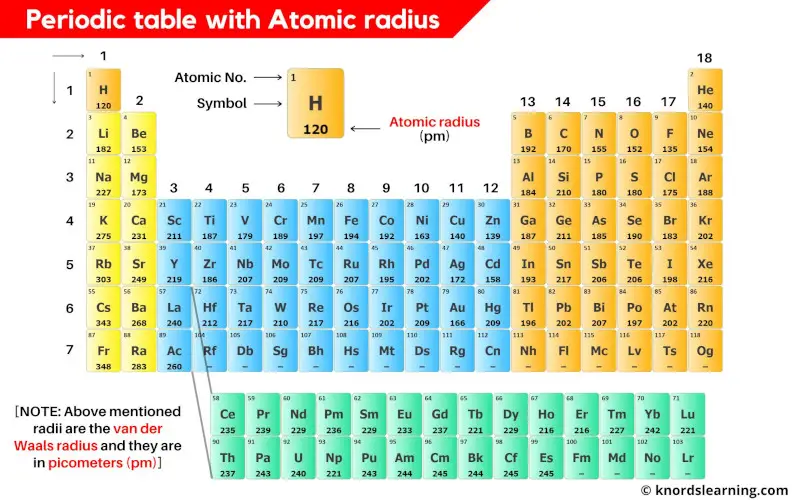
This is a periodic table with atomic radius values mentioned on it.
The above atomic radii of the elements are the van der Waals radius and they are in picometers (pm).
What is Atomic radius on a periodic table?
The atomic radius is the distance from the center of the nucleus to the outermost orbit of an atom.
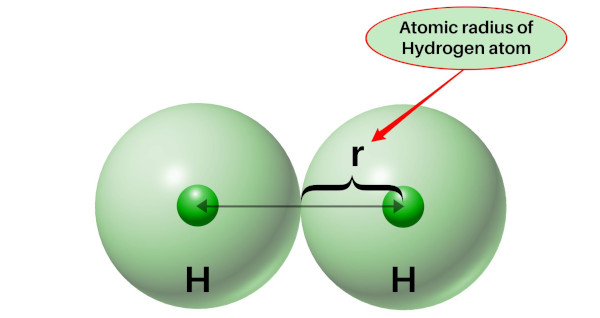
Atomic radius is measured by measuring the distance between the two atoms and then dividing the distance by 2, we get the atomic radius.
Atomic radius is measured in picometers (pm).
And, 1 pm = 10-12 meters.
Atomic radius of all the elements of periodic table
The van der Waals atomic radii of all the elements of periodic table is mentioned in the chart below.
| Atomic number | Elements | Atomic Radius of Elements (pm) |
|---|---|---|
| 1 | Atomic radius of Hydrogen (H) | 120 pm |
| 2 | Atomic radius of Helium (He) | 140 pm |
| 3 | Atomic radius of Lithium (Li) | 182 pm |
| 4 | Atomic radius of Beryllium (Be) | 153 pm |
| 5 | Atomic radius of Boron (B) | 192 pm |
| 6 | Atomic radius of Carbon (C) | 170 pm |
| 7 | Atomic radius of Nitrogen (N) | 155 pm |
| 8 | Atomic radius of Oxygen (O) | 152 pm |
| 9 | Atomic radius of Fluorine (F) | 135 pm |
| 10 | Atomic radius of Neon (Ne) | 154 pm |
| 11 | Atomic radius of Sodium (Na) | 227 pm |
| 12 | Atomic radius of Magnesium (Mg) | 173 pm |
| 13 | Atomic radius of Aluminum (Al) | 184 pm |
| 14 | Atomic radius of Silicon (Si) | 210 pm |
| 15 | Atomic radius of Phosphorus (P) | 180 pm |
| 16 | Atomic radius of Sulfur (S) | 180 pm |
| 17 | Atomic radius of Chlorine (Cl) | 175 pm |
| 18 | Atomic radius of Argon (Ar) | 188 pm |
| 19 | Atomic radius of Potassium (K) | 275 pm |
| 20 | Atomic radius of Calcium (Ca) | 231 pm |
| 21 | Atomic radius of Scandium (Sc) | 211 pm |
| 22 | Atomic radius of Titanium (Ti) | 187 pm |
| 23 | Atomic radius of Vanadium (V) | 179 pm |
| 24 | Atomic radius of Chromium (Cr) | 189 pm |
| 25 | Atomic radius of Manganese (Mn) | 197 pm |
| 26 | Atomic radius of Iron (Fe) | 194 pm |
| 27 | Atomic radius of Cobalt (Co) | 192 pm |
| 28 | Atomic radius of Nickel (Ni) | 163 pm |
| 29 | Atomic radius of Copper (Cu) | 140 pm |
| 30 | Atomic radius of Zinc (Zn) | 139 pm |
| 31 | Atomic radius of Gallium (Ga) | 187 pm |
| 32 | Atomic radius of Germanium (Ge) | 211 pm |
| 33 | Atomic radius of Arsenic (As) | 185 pm |
| 34 | Atomic radius of Selenium (Se) | 190 pm |
| 35 | Atomic radius of Bromine (Br) | 183 pm |
| 36 | Atomic radius of Krypton (Kr) | 202 pm |
| 37 | Atomic radius of Rubidium (Rb) | 303 pm |
| 38 | Atomic radius of Strontium (Sr) | 249 pm |
| 39 | Atomic radius of Yttrium (Y) | 219 pm |
| 40 | Atomic radius of Zirconium (Zr) | 186 pm |
| 41 | Atomic radius of Niobium (Nb) | 207 pm |
| 42 | Atomic radius of Molybdenum (Mo) | 209 pm |
| 43 | Atomic radius of Technetium (Tc) | 209 pm |
| 44 | Atomic radius of Ruthenium (Ru) | 207 pm |
| 45 | Atomic radius of Rhodium (Rh) | 195 pm |
| 46 | Atomic radius of Palladium (Pd) | 202 pm |
| 47 | Atomic radius of Silver (Ag) | 172 pm |
| 48 | Atomic radius of Cadmium (Cd) | 158 pm |
| 49 | Atomic radius of Indium (In) | 193 pm |
| 50 | Atomic radius of Tin (Sn) | 217 pm |
| 51 | Atomic radius of Antimony (Sb) | 206 pm |
| 52 | Atomic radius of Tellurium (Te) | 206 pm |
| 53 | Atomic radius of Iodine (I) | 198 pm |
| 54 | Atomic radius of Xenon (Xe) | 216 pm |
| 55 | Atomic radius of Caesium (Cs) | 343 pm |
| 56 | Atomic radius of Barium (Ba) | 268 pm |
| 57 | Atomic radius of Lanthanum (La) | 240 pm |
| 58 | Atomic radius of Cerium (Ce) | 235 pm |
| 59 | Atomic radius of Praseodymium (Pr) | 239 pm |
| 60 | Atomic radius of Neodymium (Nd) | 229 pm |
| 61 | Atomic radius of Promethium (Pm) | 236 pm |
| 62 | Atomic radius of Samarium (Sm) | 229 pm |
| 63 | Atomic radius of Europium (Eu) | 233 pm |
| 64 | Atomic radius of Gadolinium (Gd) | 237 pm |
| 65 | Atomic radius of Terbium (Tb) | 221 pm |
| 66 | Atomic radius of Dysprosium (Dy) | 229 pm |
| 67 | Atomic radius of Holmium (Ho) | 216 pm |
| 68 | Atomic radius of Erbium (Er) | 216 pm |
| 69 | Atomic radius of Thulium (Tm) | 227 pm |
| 70 | Atomic radius of Ytterbium (Yb) | 242 pm |
| 71 | Atomic radius of Lutetium (Lu) | 221 pm |
| 72 | Atomic radius of Hafnium (Hf) | 212 pm |
| 73 | Atomic radius of Tantalum (Ta) | 217 pm |
| 74 | Atomic radius of Tungsten (W) | 210 pm |
| 75 | Atomic radius of Rhenium (Re) | 217 pm |
| 76 | Atomic radius of Osmium (Os) | 216 pm |
| 77 | Atomic radius of Iridium (Ir) | 202 pm |
| 78 | Atomic radius of Platinum (Pt) | 209 pm |
| 79 | Atomic radius of Gold (Au) | 166 pm |
| 80 | Atomic radius of Mercury (Hg) | 209 pm |
| 81 | Atomic radius of Thallium (Tl) | 196 pm |
| 82 | Atomic radius of Lead (Pb) | 202 pm |
| 83 | Atomic radius of Bismuth (Bi) | 207 pm |
| 84 | Atomic radius of Polonium (Po) | 197 pm |
| 85 | Atomic radius of Astatine (At) | 202 pm |
| 86 | Atomic radius of Radon (Rn) | 220 pm |
| 87 | Atomic radius of Francium (Fr) | 348 pm |
| 88 | Atomic radius of Radium (Ra) | 283 pm |
| 89 | Atomic radius of Actinium (Ac) | 260 pm |
| 90 | Atomic radius of Thorium (Th) | 237 pm |
| 91 | Atomic radius of Protactinium (Pa) | 243 pm |
| 92 | Atomic radius of Uranium (U) | 240 pm |
| 93 | Atomic radius of Neptunium (Np) | 221 pm |
| 94 | Atomic radius of Plutonium (Pu) | 243 pm |
| 95 | Atomic radius of Americium (Am) | 244 pm |
| 96 | Atomic radius of Curium (Cm) | 245 pm |
| 97 | Atomic radius of Berkelium (Bk) | 244 pm |
| 98 | Atomic radius of Californium (Cf) | 245 pm |
| 99 | Atomic radius of Einsteinium (Es) | 245 pm |
| 100 | Atomic radius of Fermium (Fm) | – |
| 101 | Atomic radius of Mendelevium (Md) | – |
| 102 | Atomic radius of Nobelium (No) | – |
| 103 | Atomic radius of Lawrencium (Lr) | – |
| 104 | Atomic radius of Rutherfordium (Rf) | – |
| 105 | Atomic radius of Dubnium (Db) | – |
| 106 | Atomic radius of Seaborgium (Sg) | – |
| 107 | Atomic radius of Bohrium (Bh) | – |
| 108 | Atomic radius of Hassium (Hs) | – |
| 109 | Atomic radius of Meitnerium (Mt) | – |
| 110 | Atomic radius of Darmstadtium (Ds) | – |
| 111 | Atomic radius of Roentgenium (Rg) | – |
| 112 | Atomic radius of Copernicium (Cn) | – |
| 113 | Atomic radius of Nihonium (Nh) | – |
| 114 | Atomic radius of Flerovium (Fl) | – |
| 115 | Atomic radius of Moscovium (Mc) | – |
| 116 | Atomic radius of Livermorium (Lv) | – |
| 117 | Atomic radius of Tennessine (Ts) | – |
| 118 | Atomic radius of Oganesson (Og) | – |
External resources:
- Bondi, A. (1964, March). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), 441–451. https://doi.org/10.1021/j100785a001
- Atomic and ionic radius. (n.d.). Atomic and Ionic Radius. https://www.chemguide.co.uk/atoms/properties/atradius.html
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/trends_atomicradius.htm
- Unit 2: Atomic Theory and Bonding. (n.d.). Unit 2: Atomic Theory and Bonding. https://ch301.cm.utexas.edu/atomic/#trends/atomic-radii.html
- Atomic properties. CUNY (n.d.). http://academic.brooklyn.cuny.edu/physics/sobel/Nucphys/atomprop.html
- Atomic radius – Wikipedia. (2021, May 9). Atomic Radius – Wikipedia. https://en.wikipedia.org/wiki/Atomic_radius
- Atomic Radii. (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Atomic_Radii
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
