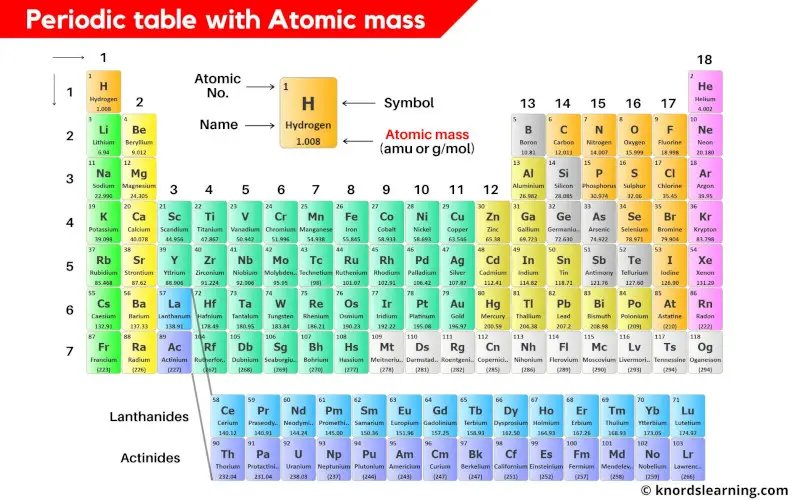
Periodic table with atomic mass and atomic number is shown in the above image.
You can also download the HD image of the periodic table with atomic mass.
(The download link is given at the end of this article.)
But before that, you can read more about some basic things about the atomic mass.
What is Atomic mass on a Periodic table?
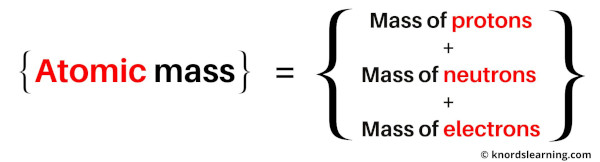
Atomic mass mentioned on the periodic table is the total mass of the single atom.
Now the atoms are made up of 3 subatomic particles.
- Protons
- Neutrons
- Electrons
So the atomic mass is the sum total of masses of protons, neutrons and electrons of an atom.
What is the unit of atomic mass?
The unit of atomic mass is amu (atomic mass unit).
1 amu is one twelfth the mass of carbon-12 atom.
1 amu = 1 g/mol = 1.66 × 10-24 grams.
Atomic mass is denoted by amu or g/mol.
Why are some atomic masses written in brackets ( )?
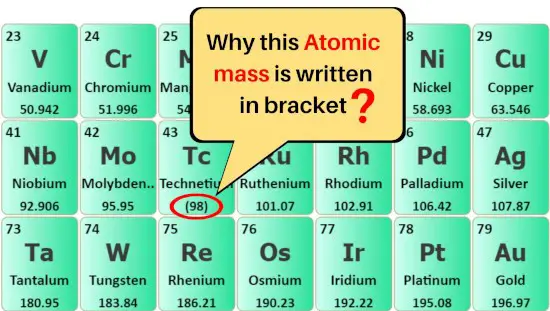
The elements whose atomic masses are written in brackets ( ) are the synthetic elements, which means these elements are artificially prepared in a laboratory.
These elements have a very short half-life and so they are very unstable.
So the atomic masses of their most stable isotope are written in the brackets ( ) in the above periodic table.
So this was some basic thing that you should know while using this periodic table.
Don’t forget to download the HD image of the periodic table with atomic mass from below.
External resources:
- Atomic Number and Atomic Mass – Radiation Emergency Medical Management. (n.d.). Atomic Number and Atomic Mass – Radiation Emergency Medical Management. https://remm.hhs.gov/atomicshorthand.htm
- P. (n.d.). Atomic Mass | Periodic Table of Elements. Atomic Mass | Periodic Table of Elements – PubChem. https://pubchem.ncbi.nlm.nih.gov/periodic-table/atomic-mass
- Atomic Weights and Isotopic Compositions for All Elements. (n.d.). Atomic Weights and Isotopic Compositions for All Elements. https://physics.nist.gov/cgi-bin/Compositions/stand_alone.pl
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Possolo, A., van der Veen, A. M. H., Meija, J., & Hibbert, D. B. (2018, January 4). Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report). Pure and Applied Chemistry, 90(2), 395–424. https://doi.org/10.1515/pac-2016-0402
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/structure_mass.htm
- Atomic Mass Data Resources | Argonne National Laboratory. (n.d.). Atomic Mass Data Resources | Argonne National Laboratory. https://www.anl.gov/phy/atomic-mass-data-resources
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

