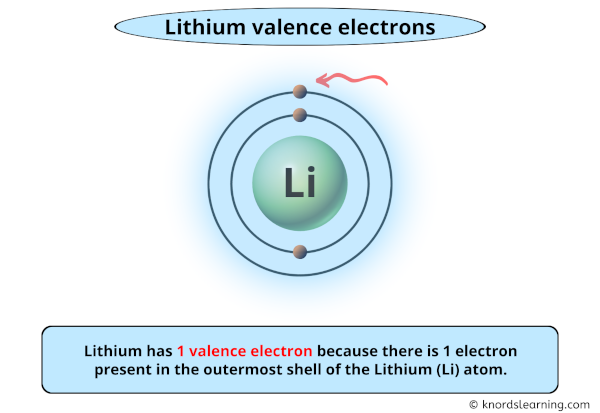
So you have seen the above image by now, right?
Awesome! You can see that lithium has 1 valence electron.
But how can you say that Lithium has 1 valence electron
+
How can you find this valence electron?
Let’s discuss this in short.
Lithium has 1 valence electron because there is 1 electron present in the outermost shell of the Lithium (Li) atom.
Now let’s see how you can easily find the valence electrons of Lithium atom (Li).
If you don’t want to read the texts, then you can also watch this video.
How to find the Valence Electrons? (2 Methods)
In order to find the valence electrons of Lithium atom (Li), you can use two methods.
Method 1: From the Periodic Table
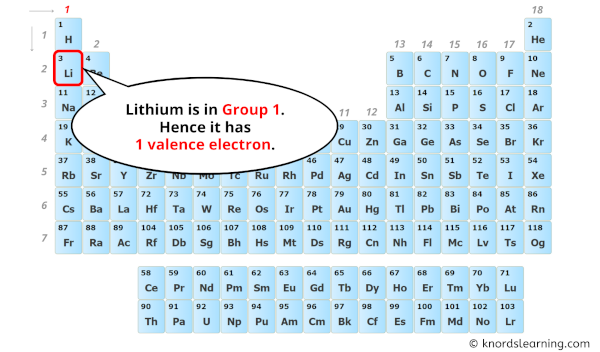
To find out the valence electrons of Lithium, you have to see the position of lithium in the periodic table.
More specifically, you have to see the group wise position of Lithium element in the periodic table.
From the above image, you can see that the Lithium (Li) is present in the group 1 of periodic table.
(Note: Group 1 is also called group 1A).
So, as the lithium element is present in group 1, it has 1 valence electron.
In this way, by knowing the position of lithium element in periodic table, you can easily find its valence electrons.
Now let’s see another method for finding the number of valence electrons in lithium.
Method 2: From the Electron Configuration
If you want to find the valence electrons of lithium from its electron configuration, then you should know its electron configuration first.
Now there are many methods to write the electron configurations, but here I will show you the easiest method, i.e by using Aufbau principle.
Aufbau principle: The Aufbau principle simply states that the orbitals with the lower energy are filled first and then the orbitals with higher energy levels are filled.
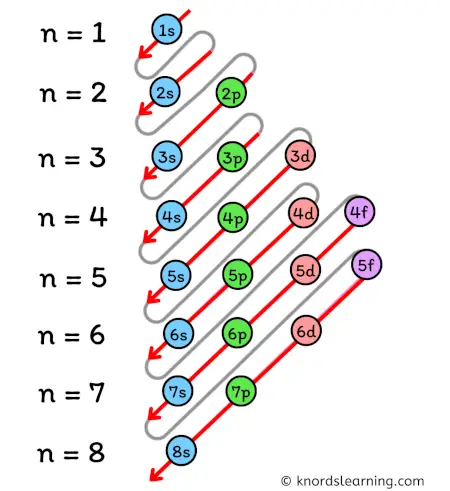
According to the Aufbau principle, the orbitals are filled in the following order:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Also the maximum number of electrons that can be accommodated in s, p, d & f orbitals are mentioned in the below table.
| Orbitals | Maximum capacity of electrons [1] |
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
Now let’s try to find the electron configuration of Lithium by using the Aufbau principle.
Electron Configuration of Lithium:
Follow the steps mentioned below to get the electron configuration of Lithium.
- To write the electron configuration of lithium, we should first know the total number of electrons present in a lithium atom.
- The lithium atom has a total of 3 electrons because its atomic number is 3 and it is a neutral atom. [2]
- Now we have to fill these 3 electrons in the atomic orbitals according to the Aufbau principle.
- According to the Aufbau principle, the electrons will be filled first in 1s orbital and then in 2s orbital.
- So in the lithium atom, the first 2 electrons will be filled in s-orbital (because s-orbital can hold only 2 electrons).
- And the remaining 1 electron will be filled in 2p orbital.
- So the electron configuration of the lithium atom is 1s2 2s1. [3]
Now in this electron configuration of lithium, we have to see the total number of electrons present in the highest energy level.
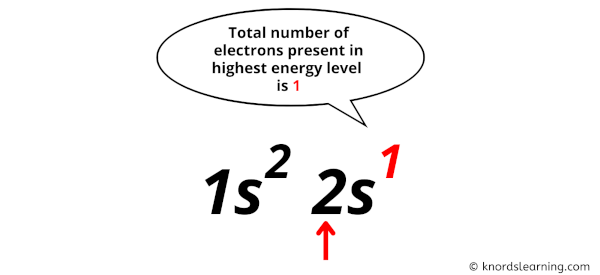
You can see in the electron configuration of lithium (1s2 2s1) that the highest energy level is 2. And the total number of electrons present in this energy level is 1.
So by knowing the electron configuration, we have found that the Lithium has 1 valence electron.
I hope you have understood the methods of finding the valence electrons in lithium.
See more related topics for your practice;
Beryllium Valence Electrons
Sodium Valence Electrons
Magnesium Valence Electrons
Potassium Valence Electrons
Calcium Valence Electrons
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
