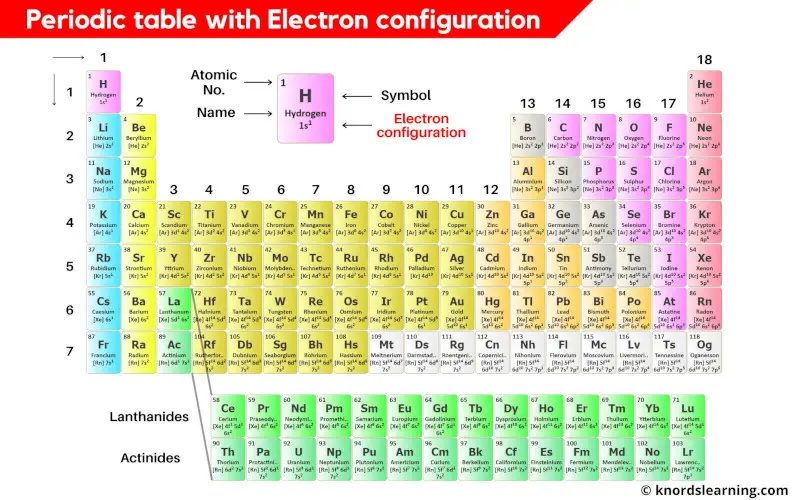
This is a periodic table with electron configuration mentioned on it.
I know you are unable to see the electron configurations in the above periodic table. So you can download the HD image.
(The download link is given at the end of this article.)
But before that, you can read more about some basic things about electron configuration of elements.
What is electron configuration on periodic table?
Electron configuration of elements is a structure which describes the arrangement of electrons in different orbits and orbitals around the nucleus.
By knowing the electron configuration of elements, we can come to know the number of electrons present in different orbits (like 1st orbit, 2nd orbit, 3rd orbit, etc).
The electron configuration also indicates the number of electrons present in different orbitals (like s-orbital, p-orbital, d-orbital and f-orbital).
List of electron configuration of elements of the periodic table
Electron configuration of the elements of periodic table is mentioned in the table below.
| Atomic no. | Element | Electron Configuration |
|---|---|---|
| 1 | Hydrogen (H) | 1s1 |
| 2 | Helium (He) | 1s2 |
| 3 | Lithium (Li) | [He] 2s1 |
| 4 | Beryllium (Be) | [He] 2s2 |
| 5 | Boron (B) | [He] 2s2 2p1 |
| 6 | Carbon (C) | [He] 2s2 2p2 |
| 7 | Nitrogen (N) | [He] 2s2 2p3 |
| 8 | Oxygen (O) | [He] 2s2 2p4 |
| 9 | Fluorine (F) | [He] 2s2 2p5 |
| 10 | Neon (Ne) | [He] 2s2 2p6 |
| 11 | Sodium (Na) | [Ne] 3s1 |
| 12 | Magnesium (Mg) | [Ne] 3s2 |
| 13 | Aluminum (Al) | [Ne] 3s2 3p1 |
| 14 | Silicon (Si) | [Ne] 3s2 3p2 |
| 15 | Phosphorus (P) | [Ne] 3s2 3p3 |
| 16 | Sulfur (S) | [Ne] 3s2 3p4 |
| 17 | Chlorine (Cl) | [Ne] 3s2 3p5 |
| 18 | Argon (Ar) | [Ne] 3s2 3p6 |
| 19 | Potassium (K) | [Ar] 4s1 |
| 20 | Calcium (Ca) | [Ar] 4s2 |
| 21 | Scandium (Sc) | [Ar] 3d1 4s2 |
| 22 | Titanium (Ti) | [Ar] 3d2 4s2 |
| 23 | Vanadium (V) | [Ar] 3d3 4s2 |
| 24 | Chromium (Cr) | [Ar] 3d5 4s1 |
| 25 | Manganese (Mn) | [Ar] 3d5 4s2 |
| 26 | Iron (Fe) | [Ar] 3d6 4s2 |
| 27 | Cobalt (Co) | [Ar] 3d7 4s2 |
| 28 | Nickel (Ni) | [Ar] 3d8 4s2 |
| 29 | Copper (Cu) | [Ar] 3d10 4s1 |
| 30 | Zinc (Zn) | [Ar] 3d10 4s2 |
| 31 | Gallium (Ga) | [Ar] 3d10 4s2 4p1 |
| 32 | Germanium (Ge) | [Ar] 3d10 4s2 4p2 |
| 33 | Arsenic (As) | [Ar] 3d10 4s2 4p3 |
| 34 | Selenium (Se) | [Ar] 3d10 4s2 4p4 |
| 35 | Bromine (Br) | [Ar] 3d10 4s2 4p5 |
| 36 | Krypton (Kr) | [Ar] 3d10 4s2 4p6 |
| 37 | Rubidium (Rb) | [Kr] 5s1 |
| 38 | Strontium (Sr) | [Kr] 5s2 |
| 39 | Yttrium (Y) | [Kr] 4d1 5s2 |
| 40 | Zirconium (Zr) | [Kr] 4d2 5s2 |
| 41 | Niobium (Nb) | [Kr] 4d4 5s1 |
| 42 | Molybdenum (Mo) | [Kr] 4d5 5s1 |
| 43 | Technetium (Tc) | [Kr] 4d5 5s2 |
| 44 | Ruthenium (Ru) | [Kr] 4d7 5s1 |
| 45 | Rhodium (Rh) | [Kr] 4d8 5s1 |
| 46 | Palladium (Pd) | [Kr] 4d10 |
| 47 | Silver (Ag) | [Kr] 4d10 5s1 |
| 48 | Cadmium (Cd) | [Kr] 4d10 5s2 |
| 49 | Indium (In) | [Kr] 4d10 5s2 5p1 |
| 50 | Tin (Sn) | [Kr] 4d10 5s2 5p2 |
| 51 | Antimony (Sb) | [Kr] 4d10 5s2 5p3 |
| 52 | Tellurium (Te) | [Kr] 4d10 5s2 5p4 |
| 53 | Iodine (I) | [Kr] 4d10 5s2 5p5 |
| 54 | Xenon (Xe) | [Kr] 4d10 5s2 5p6 |
| 55 | Caesium (Cs) | [Xe] 6s1 |
| 56 | Barium (Ba) | [Xe] 6s2 |
| 57 | Lanthanum (La) | [Xe] 5d1 6s2 |
| 58 | Cerium (Ce) | [Xe] 4f1 5d1 6s2 |
| 59 | Praseodymium (Pr) | [Xe] 4f3 6s2 |
| 60 | Neodymium (Nd) | [Xe] 4f4 6s2 |
| 61 | Promethium (Pm) | [Xe] 4f5 6s2 |
| 62 | Samarium (Sm) | [Xe] 4f6 6s2 |
| 63 | Europium (Eu) | [Xe] 4f7 6s2 |
| 64 | Gadolinium (Gd) | [Xe] 4f7 5d1 6s2 |
| 65 | Terbium (Tb) | [Xe] 4f9 6s2 |
| 66 | Dysprosium (Dy) | [Xe] 4f10 6s2 |
| 67 | Holmium (Ho) | [Xe] 4f11 6s2 |
| 68 | Erbium (Er) | [Xe] 4f12 6s2 |
| 69 | Thulium (Tm) | [Xe] 4f13 6s2 |
| 70 | Ytterbium (Yb) | [Xe] 4f14 6s2 |
| 71 | Lutetium (Lu) | [Xe] 4f14 5d1 6s2 |
| 72 | Hafnium (Hf) | [Xe] 4f14 5d2 6s2 |
| 73 | Tantalum (Ta) | [Xe] 4f14 5d3 6s2 |
| 74 | Tungsten (W) | [Xe] 4f14 5d4 6s2 |
| 75 | Rhenium (Re) | [Xe] 4f14 5d5 6s2 |
| 76 | Osmium (Os) | [Xe] 4f14 5d6 6s2 |
| 77 | Iridium (Ir) | [Xe] 4f14 5d7 6s2 |
| 78 | Platinum (Pt) | [Xe] 4f14 5d9 6s1 |
| 79 | Gold (Au) | [Xe] 4f14 5d10 6s1 |
| 80 | Mercury (Hg) | [Xe] 4f14 5d10 6s2 |
| 81 | Thallium (Tl) | [Xe] 4f14 5d10 6s2 6p1 |
| 82 | Lead (Pb) | [Xe] 4f14 5d10 6s2 6p2 |
| 83 | Bismuth (Bi) | [Xe] 4f14 5d10 6s2 6p3 |
| 84 | Polonium (Po) | [Xe] 4f14 5d10 6s2 6p4 |
| 85 | Astatine (At) | [Xe] 4f14 5d10 6s2 6p5 |
| 86 | Radon (Rn) | [Xe] 4f14 5d10 6s2 6p6 |
| 87 | Francium (Fr) | [Rn] 7s1 |
| 88 | Radium (Ra) | [Rn] 7s2 |
| 89 | Actinium (Ac) | [Rn] 6d1 7s2 |
| 90 | Thorium (Th) | [Rn] 6d2 7s2 |
| 91 | Protactinium (Pa) | [Rn] 5f2 6d1 7s2 |
| 92 | Uranium (U) | [Rn] 5f3 6d1 7s2 |
| 93 | Neptunium (Np) | [Rn] 5f4 6d1 7s2 |
| 94 | Plutonium (Pu) | [Rn] 5f6 7s2 |
| 95 | Americium (Am) | [Rn] 5f7 7s2 |
| 96 | Curium (Cm) | [Rn] 5f7 6d1 7s2 |
| 97 | Berkelium (Bk) | [Rn] 5f9 7s2 |
| 98 | Californium (Cf) | [Rn] 5f10 7s2 |
| 99 | Einsteinium (Es) | [Rn] 5f11 7s2 |
| 100 | Fermium (Fm) | [Rn] 5f12 7s2 |
| 101 | Mendelevium (Md) | [Rn] 5f13 7s2 |
| 102 | Nobelium (No) | [Rn] 5f14 7s2 |
| 103 | Lawrencium (Lr) | [Rn] 5f14 7s2 7p1 |
| 104 | Rutherfordium (Rf) | [Rn] 5f14 6d2 7s2 |
| 105 | Dubnium (Db) | [Rn] 5f14 6d3 7s2 |
| 106 | Seaborgium (Sg) | [Rn] 5f14 6d4 7s2 |
| 107 | Bohrium (Bh) | [Rn] 5f14 6d5 7s2 |
| 108 | Hassium (Hs) | [Rn] 5f14 6d6 7s2 |
| 109 | Meitnerium (Mt) | [Rn] 5f14 6d7 7s2 |
| 110 | Darmstadtium (Ds) | [Rn] 5f14 6d8 7s2 |
| 111 | Roentgenium (Rg) | [Rn] 5f14 6d9 7s2 |
| 112 | Copernicium (Cn) | [Rn] 5f14 6d10 7s2 |
| 113 | Nihonium (Nh) | [Rn] 5f14 6d10 7s2 7p1 |
| 114 | Flerovium (Fl) | [Rn] 5f14 6d10 7s2 7p2 |
| 115 | Moscovium (Mc) | [Rn] 5f14 6d10 7s2 7p3 |
| 116 | Livermorium (Lv) | [Rn] 5f14 6d10 7s2 7p4 |
| 117 | Tennessine (Ts) | [Rn] 5f14 6d10 7s2 7p5 |
| 118 | Oganesson (Og) | [Rn] 5f14 6d10 7s2 7p6 |
Don’t forget to download the HD image of the periodic table with electron configuration from below.
External resources:
- 2.4 Electron Configurations. (2016, May 13). Chemistry LibreTexts. https://chem.libretexts.org/Courses/Valley_City_State_University/Chem_115/Chapter_2%3A_Atomic_Structure/2.4_Electron_Configurations
- Electron configuration – Wikipedia. (2018, June 12). Electron Configuration – Wikipedia. https://en.wikipedia.org/wiki/Electron_configuration
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Electronic structure of the elements. (2000, March). The European Physical Journal C, 15(1–4), 78–79. https://doi.org/10.1007/bf02683401
- Electron Configurations. (n.d.). Electron Configurations. https://www.chem.fsu.edu/chemlab/chm1045/e_config.html
- Electron Configurations & The Periodic Table. (n.d.). Electron Configurations & the Periodic Table. https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/intro2.htm
- How to Write Electron Configurations. (n.d.). How to Write Electron Configurations. https://terpconnect.umd.edu/~wbreslyn/chemistry/electron-configurations/index.html
- Unit 2: Atomic Theory and Bonding. (n.d.). Unit 2: Atomic Theory and Bonding. https://ch301.cm.utexas.edu/atomic/#e-config/electron-config.html
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
