Molar mass of AgCl (Silver chloride) is 143.32 g/mol.
Well, now you have come to know the molar mass of AgCl.
But how can you get this value?
Let me show you the calculation to get the molar mass of AgCl (Silver chloride).
If you are a visual learner like me, then here is a short one minute video for you.
AgCl (Silver chloride) Molar Mass Calculation
If you have a periodic table with you, then you can easily calculate the molar mass of AgCl (Silver chloride).
Because the molar mass of any molecule (or compound) can be calculated by simply adding the molar masses of individual atoms.
Now here we have to find the molar mass of AgCl (Silver chloride).
So for that, have a look at the periodic table given below.
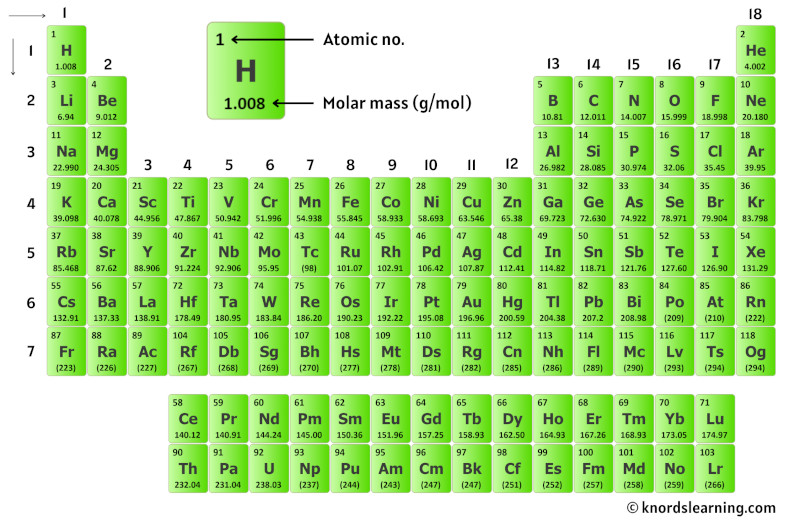
You can see the molar mass value of all the atoms from this periodic table.
Now in AgCl, there is 1 Silver atom and 1 Chlorine atom.
So let’s look at the molar mass of Silver and Chlorine from the above periodic table.
You can see that;
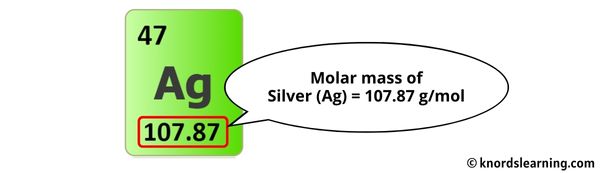
The molar mass of Silver is 107.87 g/mol. [1]
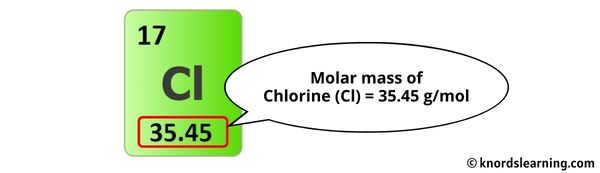
The molar mass of Chlorine is 35.45 g/mol. [2]
Now, to calculate the molar mass of AgCl, you just have to add the molar mass of all the individual atoms that are present in AgCl.
You can see that in AgCl, there is 1 Silver atom and 1 Chlorine atom.
So, Molar mass of AgCl = Molar mass of 1 Silver (Ag) atom + Molar mass of 1 Chlorine (Cl) atom.
= 107.87 + 35.45
= 143.32 g/mol
Hence the Molar mass of AgCl is 143.32 g/mol.
I hope you have understood the short and simple calculation for finding the molar mass of AgCl.
Remember
- In some books, you may see the unit of molar mass as grams/mole or g/mole. But all these units (i.e g/mol, grams/mole and g/mole) are the same.
- Always follow the calculation order to avoid any mistakes in calculation. First solve the brackets, then multiplications and at last do the final addition.
- And don’t forget to put the unit g/mol to your final calculated molar mass.
Check out other related topics for more practice;
Fe2O3 Molar Mass
I2 Molar Mass
KBr (Potassium bromide) Molar Mass
F2 Molar Mass
H2O2 (Hydrogen peroxide) Molar Mass
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.