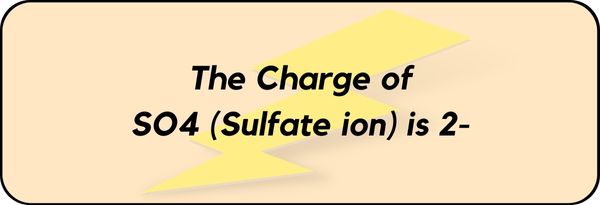
The Charge of SO4 (Sulfate ion) is 2-.
But the question is how can you find the charge on SO4 (sulfate ion)?
Well there are 2 methods by which you can find the charge of SO4.
Lets dive right into these methods one by one.
If you are a visual learner like me, then here is a short two minute video for you.
Method 1: By looking at what it is bonded to
The charge of SO4 (Sulfate ion) can be found out by looking at what it is bonded to.
So let’s take some examples of compounds that contain SO4; like H2SO4, Na2SO4, etc.
Example 1: H2SO4
In H2SO4, the SO4 is bonded to Hydrogen (H).
You know that the ionic charge of H is 1+.
So you can easily say that the charge of SO4 should be 2-, then only it will get canceled out.
Hence the charge of SO4 in H2SO4 is 2-.
Example 2: Na2SO4
In Na2SO4, the SO4 is bonded to Sodium (Na).
And again, you know that the ionic charge of Na is 1+.
So here also you can easily say that the charge of SO4 should be 2-, then only it will get canceled out.
Hence the charge of SO4 in Na2SO4 is 2-.
As seen from the above examples,
The charge of SO4 is 2-.
In this way, you can easily find the charge of SO4 by looking at what it is bonded to.
Method 2: By calculating the formal charge using lewis structure
In order to calculate the formal charge on SO4 (Sulfate ion), you should know the Lewis dot structure of SO4 (Sulfate ion).
Here is the lewis structure of SO4.
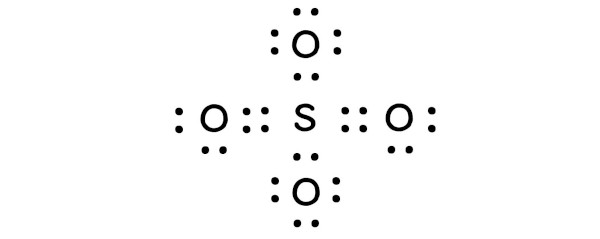
Now using the above lewis structure of SO4, you have to find the formal charge on each atom that is present in the SO4 molecule.
For calculating the formal charge, you need to remember this formula;
Formal charge = Valence electrons – Nonbonding electrons – (Bonding electrons)/2
You can see the bonding and nonbonding electrons of SO4 from the image given below.
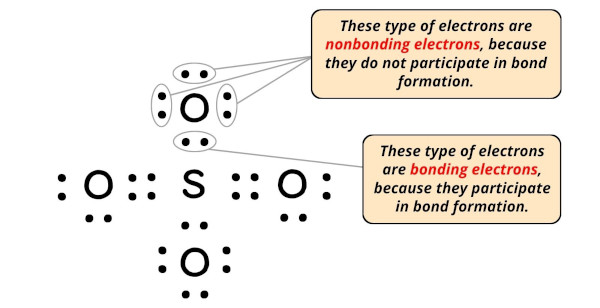
So now let’s calculate the formal charge on each individual atom present in SO4.
Formal charge on Sulfur atom:
Valence electrons = 6 (as it is in group 16 on periodic table) [1]
Nonbonding electrons = 0
Bonding electrons = 12
So according to the formula of formal charge, you will get;
Formal charge on Sulfur = Valence electrons – Nonbonding electrons – (Bonding electrons)/2 = 6 – 0 – (12/2) = 0

So the formal charge on sulfur atom is 0.
Formal charge on double bonded Oxygen:
Valence electron = 6 (as it is in group 16 on periodic table) [2]
Nonbonding electrons = 4
Bonding electrons = 4
So according to the formula of formal charge, you will get;
Formal charge on double bonded Oxygen = Valence electrons – Nonbonding electrons – (Bonding electrons)/2 = 6 – 4 – (4/2) = 0

So the formal charge on double bonded oxygen atom is 0.
Formal charge on single bonded Oxygen:
Valence electron = 6 (as it is in group 16 on periodic table)
Nonbonding electrons = 6
Bonding electrons = 2
So according to the formula of formal charge, you will get;
Formal charge on single bonded Oxygen = Valence electrons – Nonbonding electrons – (Bonding electrons)/2 = 6 – 6 – (2/2) = 1-

So the formal charge on single bonded oxygen atom is 1-.
Now let’s put all these charges on the lewis dot structure of SO4.
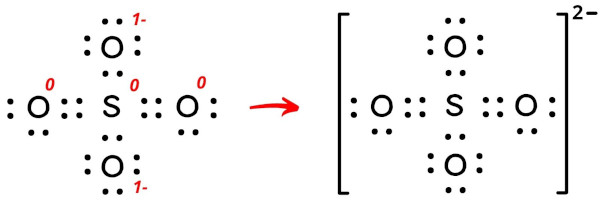
So there is overall 2- charge left on the entire molecule.
This indicates that the SO4 (Sulfate ion) has 2- charge.
I hope you have understood the above calculations of SO4 (Sulfate ion). But for your tests, you don’t need to remember the entire calculations. You should just try to remember that SO4 has 2- charge.
Check out some other related topics for your practice.
Related topics:
Charge of Iodine (I)
Charge on CO3 (Carbonate ion)
Charge of Beryllium (Be)
Charge of Sulfur (S)
Charge on PO4 (Phosphate ion)
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.