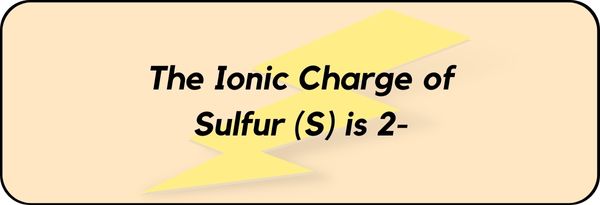
The Ionic Charge of Sulfur (S) is 2-.
But the question is how can you find the ionic charge on Sulfur (S)?
Well there are 2 methods by which you can find the ionic charge of Sulfur (S).
Lets dive right into these methods one by one.
If you are a visual learner like me, then here is a short one minute video for you.
Method 1: By looking at the ionic charge trends on periodic table
This is a very simple method to determine the ionic charge of the monatomic ions.
First of all, let’s look at the group-wise ionic charge trends on the periodic table.
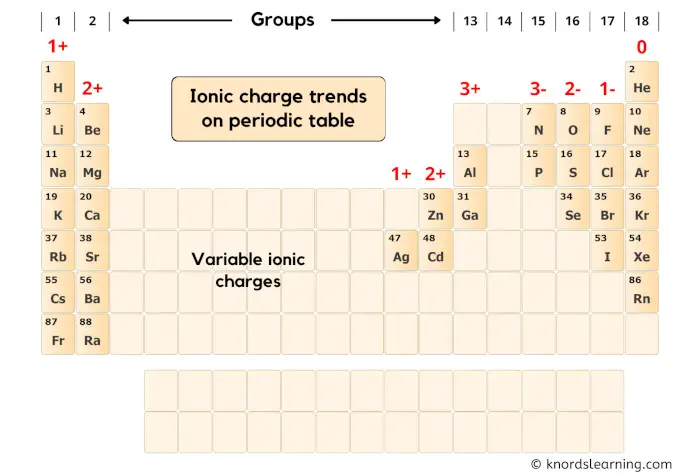
You can see in the above periodic table that;
- Elements of group 1 have 1+ ionic charge,
- Elements of group 2 have 2+ ionic charge,
(Exclude transition metals, because they have variable ionic charges)
- Elements of group 13 have 3+ ionic charge,
(Exclude group 14, because they can have 4+ or 4- ionic charges)
- Elements of group 15 have 3- ionic charge,
- Elements of group 16 have 2- ionic charge,
- Elements of group 17 have 1- ionic charge,
- Elements of group 18 have 0 ionic charge,
Now here our element is Sulfur (S) which lies in group 16 of the periodic table.
Hence the ionic charge of Sulfur (S) is 2-.
Note:
The blank area shown in the above periodic table are mostly the transition and post-transition elements.
They show variable ionic charge.
Hence we cannot find their ionic charge by simply looking at the periodic table.
In order to find the charge of these elements, we need to look at what it is bonded with.
For more information, read the example of finding the charge of Copper.
Okay so now let’s see another method to find the ionic charge of Sulfur (S).
Method 2: By using electron configuration
The electron configuration of Sulfur is: 1s2 2s2 2p6 3s2 3p4 or [Ne] 3s2 3p4. [1]
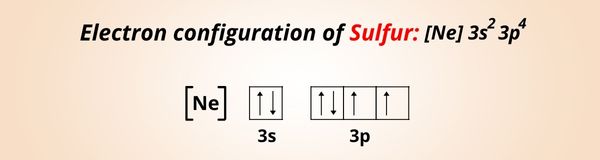
You can see that the outermost orbit of Sulfur has 6 electrons.
During the chemical reaction, sulfur gains 2 more electrons and achieves the nearest noble gas configuration to become stable.
And as the Sulfur (S) gains 2 electrons, it forms S2- ion.
Hence the ionic charge of Sulfur (S) is 2-.
I hope you have understood the reason behind the 2- charge of sulfur.
Check out some other related topics for your practice.
Related topics:
Charge on PO4 (Phosphate ion)
Charge of Silver (Ag)
Charge of Zinc (Zn)
Charge of Aluminum (Al)
Charge of Sodium (Na)
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.