CH2F2 is a POLAR molecule.
But why?
And how can you say that CH2F2 is a polar molecule?
Want to know the reason?
Let’s dive into it!
CH2F2 is a POLAR molecule because the C-F bonds present in the molecule are polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire CH2F2 molecule polar.
Let me explain this in detail with the help of CH2F2 lewis structure and its 3D geometry.
Why is CH2F2 a Polar molecule? (Explained in 2 Steps)
CH2F2 is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it.
Let me explain this to you in 2 steps!
Step #1: Draw the lewis structure
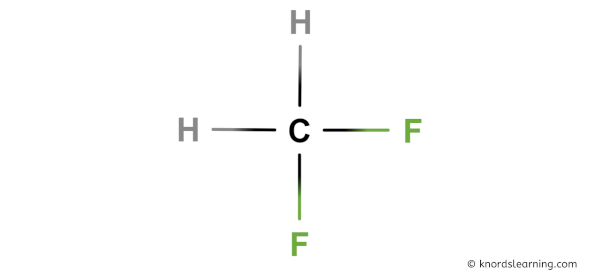
Here is a skeleton of CH2F2 lewis structure and it contains two C-H bonds and two C-F bonds.
(Note: If you want to know the steps of drawing the CH2F2 lewis dot structure, then visit this article: CH2F2 lewis structure, Or you can also watch this short 2 minute video).
So from the above diagram we have come to know that the CH2F2 molecule has two C-H bonds and two C-F bonds.
Now in the next step we have to check whether these two C-H bonds and two C-F bonds are polar or nonpolar.
And we also have to check the molecular geometry of CH2F2.
Step #2: Check the bond polarity and molecular geometry
The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the two atoms.
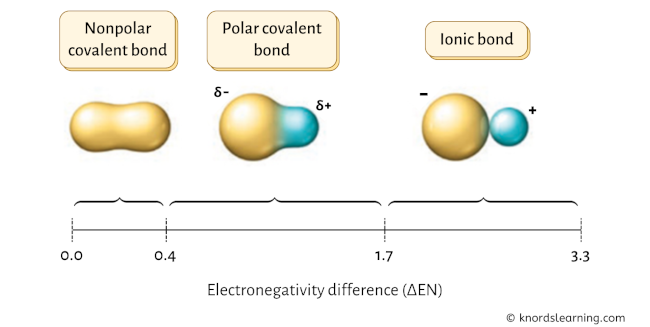
Have a look at the above image.
- If the electronegativity difference (ΔEN) is less than 0.4, then the bond is nonpolar covalent bond.
- If the electronegativity difference (ΔEN) is between 0.4 to 1.7, then the bond is polar covalent bond.
- If the electronegativity difference (ΔEN) is greater than 1.7, then the bond is an ionic bond. [1] [2] [3] [4]
Now let’s come to the example of CH2F2 molecule. It has two C-H bonds and two C-F bonds.
You can see the electronegativity values of Carbon (C), Hydrogen (H) and Fluorine (F) atoms from the periodic table given below.
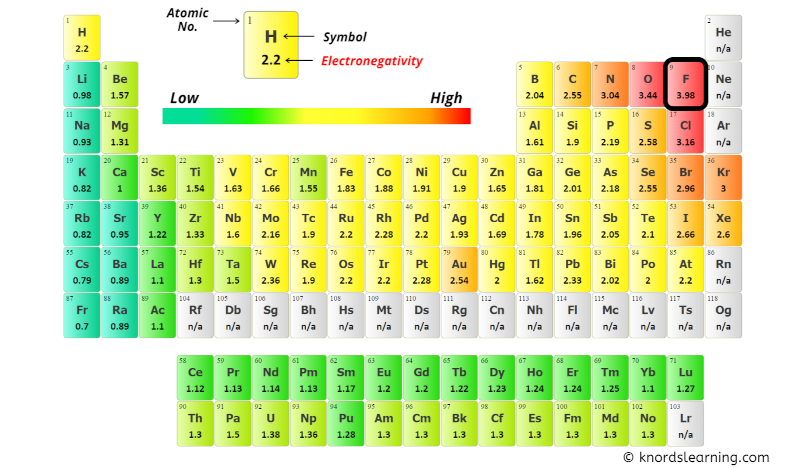
From the above image;
- Electronegativity of Carbon (C) = 2.55 [5]
- Electronegativity of Hydrogen (H) = 2.2 [6]
- Electronegativity of Fluorine (F) = 3.98 [7]
Now let’s see the polarity of each bond.
For C-H bond;
The electronegativity difference (ΔEN) = 2.55 – 2.2 = 0.35
This value is less than 0.4, which indicates that the bond between Carbon (C) and Hydrogen (H) is nonpolar.
Hence, each C-H bond is a nonpolar covalent bond.
For C-F bond;
The electronegativity difference (ΔEN) = 3.98 – 2.55 = 1.43
This value lies between 0.4 to 1.7, which indicates that the bond between Carbon (C) and Fluorine (F) is polar.
Hence, each C-F bond is a polar covalent bond.
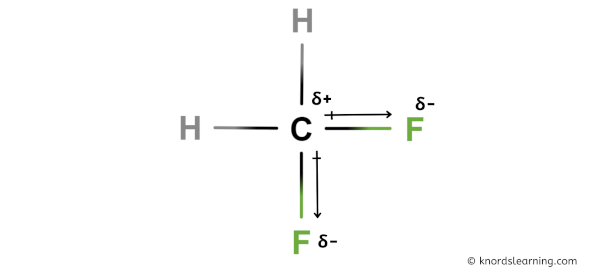
You can see in the above image that because of large electronegativity difference of Carbon and Fluorine atoms, the partial positive charge (ẟ+) appears on the Carbon atom (C) and partial negative charge (ẟ-) appears on the Fluorine atoms (F).
From this, you can easily get the idea that the CH2F2 molecule is a polar molecule.
But let’s also see its 3D molecular geometry for better understanding.
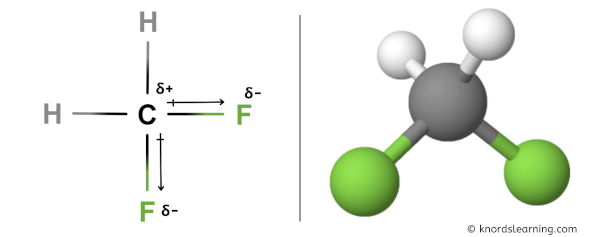
Have a look at this 3D structure of CH2F2. The more electronegative fluorine atom (F) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on carbon atom (C) and partial negative charge on fluorine atoms (F).
Because of this, there are positive and negative poles of charges on the overall molecule of CH2F2.
Hence, the CH2F2 molecule is a polar molecule.
I hope you have understood the reason behind the polar nature of CH2F2 molecule.
See the polarity of other molecules to make your concepts clear:
Is SiCl4 Polar or Nonpolar?
Is F2 Polar or Nonpolar?
Is I2 Polar or Nonpolar?
Is H2O2 Polar or Nonpolar?
Is Ethyl acetate (C4H8O2) Polar or Nonpolar?
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.