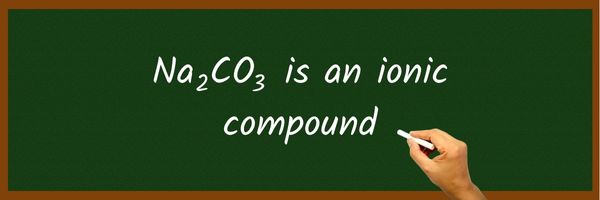
Na2CO3 is an ionic compound because it is formed by two ions, Na2+ and CO32-. These positive and negative ions produce the force of attraction between them which results in an ionic bond.
Moreover when the metal combines with nonmetal, it usually forms an ionic compound. Here, Na is a metal and CO3 is a group of nonmetals. So when they combine, it forms an ionic compound.
Well, now you have got to know that Na2CO3 is an ionic compound, but let me explain the in-depth reason why Na2CO3 is an ionic compound.
If you are a visual learner like me, then here is a short two minute video for you.
Why is Na2CO3 an ionic compound?
As mentioned above, you can simply remember that when the metal combines with nonmetal, the bond between them is an ionic bond.
Here in Na2CO3, the Na atom is a metal and the CO3 is a group of nonmetals.

Hence the bond between them is an ionic bond.
But how does the ionic bond form between Na and CO3?
When Na and CO3 combine with each other, the electron transfer takes place from Na to CO3.
In other words, each Na atom loses 1-1 electrons and the CO3 gains these 2 electrons.
Due to this, the Sodium becomes a positive ion (Na+) and CO3 becomes a negative ion (CO3)2-.
Now because of the positive charge of Sodium ion and negative charge of CO3 ion, the electrostatic force of attraction is produced between them.
This electrostatic force between Na ions and CO3 ion results in an ionic bond between them.
Hence, Na2CO3 is an ionic compound.
I hope you have understood the reason why Na2CO3 is an ionic compound.
Check out other compounds to see whether they are ionic or covalent;
Is N2 Ionic or Covalent?
Is NaF Ionic or Covalent?
Is PCl3 Ionic or Covalent?
Is SF6 (Sulfur hexafluoride) Ionic or Covalent?
Is SO3 Ionic or Covalent?
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.