
SF6 (Sulfur hexafluoride) is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, S is a nonmetal and F is also a nonmetal. So when they combine, it forms a covalent compound.
Well, now you have got to know that SF6 is a covalent compound, but let me explain the in-depth reason why SF6 is a covalent compound.
If you are a visual learner like me, then here is a short one minute video for you.
Why is SF6 a Covalent compound?
As mentioned above, you can simply remember that when the nonmetal combines with another nonmetal, the bond between them is a covalent bond.
Here in SF6, the S atom is a nonmetal and the F atom is also a nonmetal.

Hence the bond between them is a covalent bond.
Is SF6 polar covalent or nonpolar covalent?
In order to know whether SF6 is a polar covalent molecule or nonpolar covalent molecule, we have to check the electronegativity difference of the combining atoms.
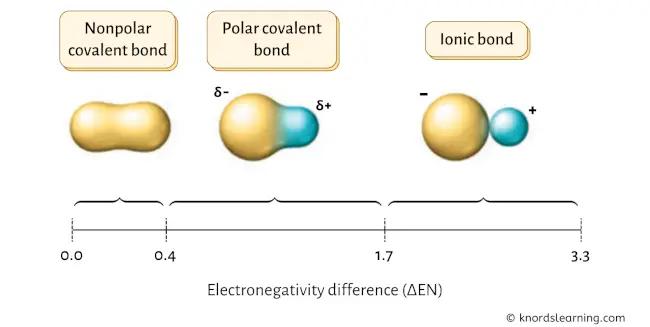
If the electronegativity difference (ΔEN) is less than 0.4, then the bond is nonpolar covalent bond.
If the electronegativity difference (ΔEN) is between 0.4 to 1.7, then the bond is polar covalent bond. [1] [2] [3] [4] [5]
Now the electronegativity of Sulfur and Fluorine are mentioned below. (You can see the electronegativity of all the elements from this electronegativity chart).
- Electronegativity of Sulfur (S) = 2.58
- Electronegativity of Fluorine (F) = 3.98
So for SF6, the electronegativity difference (ΔEN) = 3.98 – 2.58 = 1.4
This value lies between 0.4 to 1.7, which indicates that the bond between Sulfur (S) and Fluorine (F) is polar covalent bond.
But if you look at the 3D structure of SF6, you can see that the structure of SF6 is symmetrical.
As the bonds (S-F) are symmetrical and the SF6 molecule has a symmetrical geometry, their bond polarity gets canceled with each other.
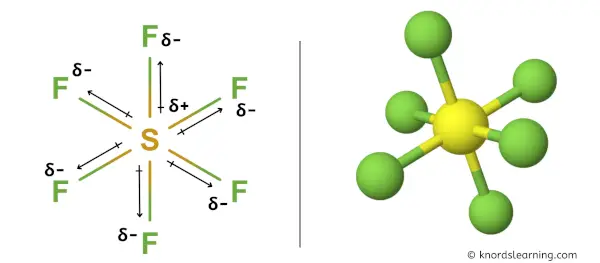
Hence, SF6 is a nonpolar covalent molecule.
I hope you have understood the reason why SF6 is a nonpolar covalent compound.
Check out other compounds to see whether they are ionic or covalent;
Is SO3 Ionic or Covalent?
Is CaF2 Ionic or Covalent?
Is K2O Ionic or Covalent?
Is KBr Ionic or Covalent?
Is KI Ionic or Covalent?
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

