
OCS is a POLAR molecule.
But why?
And how can you say that OCS is a polar molecule?
Want to know the reason?
Let’s dive into it!
OCS (or COS) is a POLAR molecule because the Oxygen-Carbon bond present in the molecule is polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire OCS molecule polar.
Let me explain this in detail with the help of OCS lewis structure and its 3D geometry.
Why is OCS a Polar molecule? (Explained in 2 Steps)
OCS is a polar molecule because it has poles of partial positive charge (ẟ+) and partial negative charge (ẟ-) on it.
Let me explain this to you in 2 steps!
Step #1: Draw the lewis structure
Here is a skeleton of OCS lewis structure and it contains one Carbon-Oxygen bond and one Carbon-Sulfur bond.
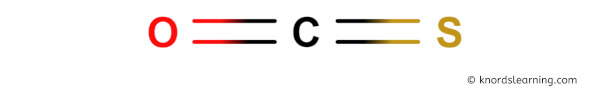
(Note: If you want to know the steps of drawing the OCS lewis dot structure, then visit this article: OCS lewis structure, Or you can also watch this short 2 minute video).
So from the above diagram we have come to know that the OCS molecule has one Carbon-Oxygen bond and one Carbon-Sulfur bond.
Now in the next step we have to check whether the Carbon-Oxygen bond and Carbon-Sulfur bond are polar or nonpolar.
And we also have to check the molecular geometry of OCS.
Step #2: Check the bond polarity and molecular geometry
The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (ΔEN) between the two atoms.
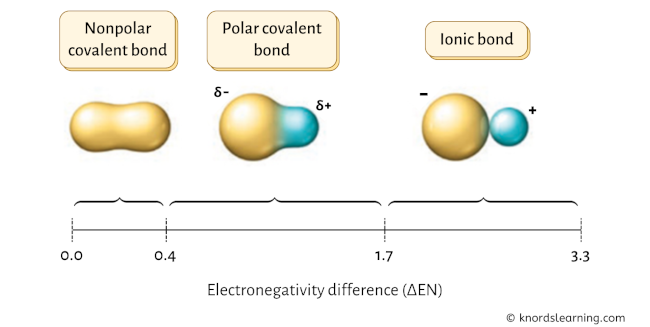
Have a look at the above image.
- If the electronegativity difference (ΔEN) is less than 0.4, then the bond is nonpolar covalent bond.
- If the electronegativity difference (ΔEN) is between 0.4 to 1.7, then the bond is polar covalent bond.
- If the electronegativity difference (ΔEN) is greater than 1.7, then the bond is an ionic bond. [1] [2] [3] [4] [5]
Now let’s come to the example of an OCS molecule. It has one Carbon-Oxygen bond and one Carbon-Sulfur bond.
You can see the electronegativity values of Oxygen (O), Carbon (C) and Sulfur (S) atoms from the periodic table given below.
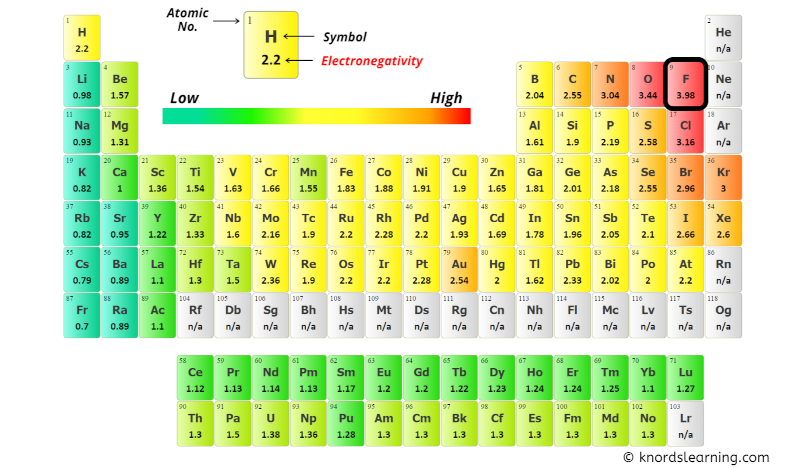
From the above image;
- Electronegativity of Oxygen (O) = 3.44 [6]
- Electronegativity of Carbon (C) = 2.55 [7]
- Electronegativity of Sulfur (S) = 2.58 [8]
Now let’s see the polarity of each bond.
For Carbon-Oxygen bond;
The electronegativity difference (ΔEN) = 3.44 – 2.55 = 0.89
This value lies between 0.4 to 1.7, which indicates that the bond between Carbon (C) and Oxygen (O) is polar.
Hence, the Carbon-Oxygen bond is a polar covalent bond.
For Carbon-Sulfur bond;
The electronegativity difference (ΔEN) = 2.58 – 2.55 = 0.03
This value is less than 0.4, which indicates that the bond between Carbon (C) and Sulfur (S) is nonpolar.
Hence, the Carbon-Sulfur bond is a nonpolar covalent bond.
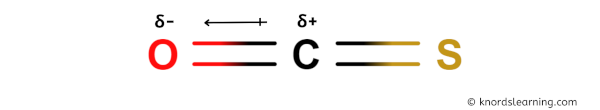
You can see in the above image that because of large electronegativity difference of Carbon and Oxygen atom, the partial positive charge (ẟ+) appears on the Carbon atom (C) and partial negative charge (ẟ-) appears on the Oxygen atom (O).
From this, you can easily get the idea that the OCS molecule is a polar molecule.
But let’s also see its 3D molecular geometry for better understanding.

Have a look at this 3D structure of OCS. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on carbon atom (C) and partial negative charge on oxygen atom (O).
Because of this, there are positive and negative poles of charges on the overall molecule of OCS.
Hence, the OCS molecule is a polar molecule.
I hope you have understood the reason behind the polar nature of OCS molecule.
See the polarity of other molecules to make your concepts clear:
Is Benzene (C6H6) Polar or Nonpolar?
Is SiH4 Polar or Nonpolar?
Is Toluene (C6H5CH3) Polar or Nonpolar?
Is PBr5 Polar or Nonpolar?
Is SiO2 Polar or Nonpolar?
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
