
I’m super excited to teach you the lewis structure of F2 in just 6 simple steps.
Infact, I’ve also given the step-by-step images for drawing the lewis dot structure of F2 molecule.
So, if you are ready to go with these 6 simple steps, then let’s dive right into it!
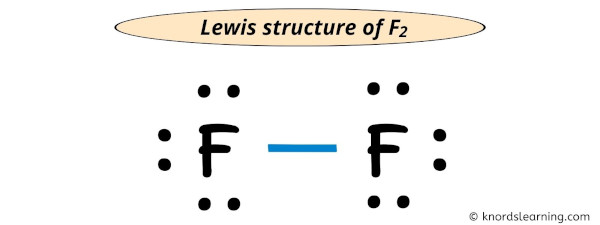
Lewis structure of F2 (Fluorine) contains one single bond between both the Fluorine (F) atoms. And both the Fluorine atoms have three lone pairs on it.
Let’s draw and understand this lewis dot structure step by step.
(Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of F2).
6 Steps to Draw the Lewis Structure of F2
Step #1: Calculate the total number of valence electrons
Here, the given molecule is F2 (Fluorine). In order to draw the lewis structure of F2, first of all you have to find the total number of valence electrons present in the F2 molecule.
(Valence electrons are the number of electrons present in the outermost shell of an atom).
So, let’s calculate this first.
Calculation of valence electrons in F2
- For Fluorine:
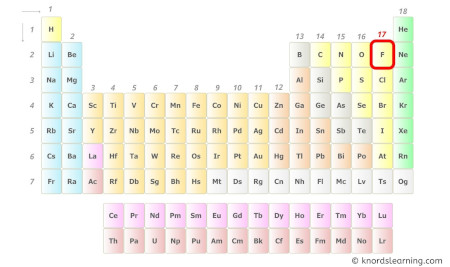
Fluorine is a group 17 element on the periodic table. [1]
Hence, the valence electrons present in fluorine is 7 (see below image).

Hence, total number of Valence electrons in F2 molecule = 7(2) = 14
Step #2: Select the center atom
While selecting the atom, you have to put the least electronegative atom at the center.
But here in the F2 molecule, both the atoms are same. So you can consider any of the atoms as a center atom.

So, let’s assume that the fluorine which is on the right side is the central atom.
Step #3: Put two electrons between the atoms to represent a chemical bond
Now in the above sketch of F2 molecule, put the two electrons (i.e electron pair) between both the fluorine atoms to represent a chemical bond between them.

These pair of electrons present between the Fluorine (F) atoms form a chemical bond, which bonds both the fluorine atoms with each other in a F2 molecule.
Step #4: Complete the octet (or duplet) on outside atom. If the valence electrons are left, then put the valence electrons pair on the central atom
Don’t worry, I’ll explain!
In the Lewis structure of F2, we have just assumed the right side fluorine atom as a central atom and so the left side fluorine atom is an outer atom.
So now, we have to complete the octet on this left side fluorine atom.
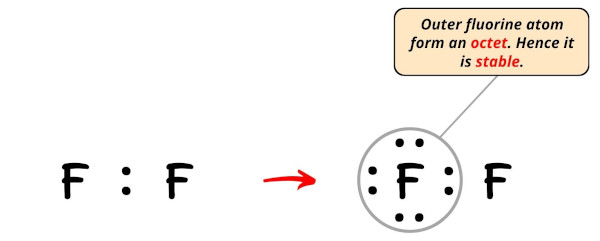
Now, you can see in the above image that the outer fluorine atom forms an octet.
Also, only 8 valence electrons of F2 molecule are used in the above structure.
But there are total 14 valence electrons in F2 molecule (as calculated in step #1).
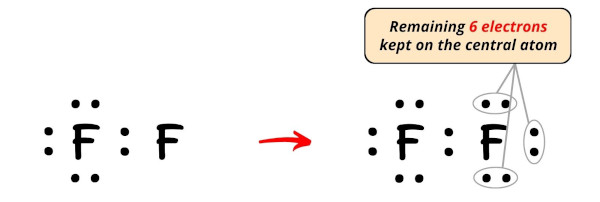
So the number of electrons left to be kept on the central atom = 14 – 8 = 6.
So let’s keep these six electrons (i.e 3 electron pairs) on the central atom (i.e right side fluorine atom).
Now, let’s move to the next step.
Step #5: Check whether the central atom has octet or not
In this step, we have to check whether the central atom (i.e right side fluorine atom) has an octet or not.
In simple words, we have to check whether the central Fluorine (F) atom is having 8 electrons or not.
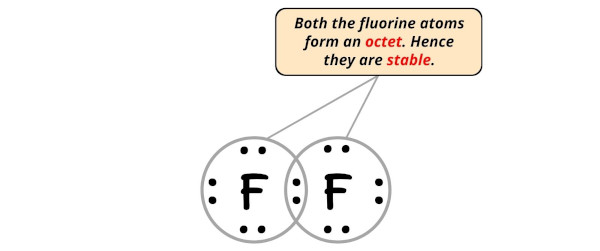
As you can see from the above image, the central atom (i.e right side fluorine atom) has 8 electrons. So it fulfills the octet rule and this fluorine atom is also stable.
Step #6: Final step – Check the stability of lewis structure by calculating the formal charge on each atom
Now, you have come to the final step and here you have to check the formal charge on the fluorine atoms (F).
For that, you need to remember the formula of formal charge;
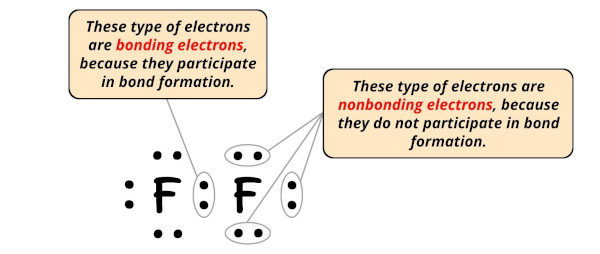
Formal charge = Valence electrons – Nonbonding electrons – (Bonding electrons)/2
- For Fluorine:
Valence electron = 7 (as it is in group 17)
Nonbonding electrons = 6
Bonding electrons = 2
| Formal charge | = | Valence electrons | – | Nonbonding electrons | – | (Bonding electrons)/2 | ||
| F | = | 7 | – | 6 | – | 2/2 | = | 0 |
So you can see above that the formal charge on both the fluorine atoms are “zero”.
Hence, there will not be any change in the above structure and the above lewis structure of F2 is the final stable structure only.
Each electron pair (:) in the lewis dot structure of F2 represents the single bond ( | ). So the above lewis dot structure of F2 can also be represented as shown below.
Related lewis structures for your practice:
Lewis structure of CH2Cl2
Lewis structure of ClO2-
Lewis structure of ClO3-
Lewis structure of HCl
Lewis structure of H2
Article by;
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
