Ready to learn how to draw the lewis structure of H2?
Awesome!
Here, I have explained simple steps to draw the lewis dot structure of H2 (along with images).
So let’s dive right into it!
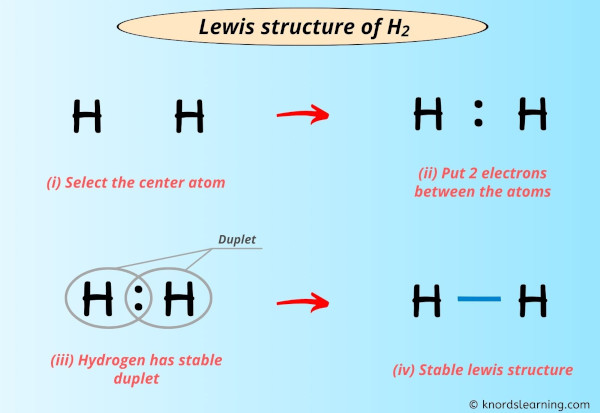
Lewis structure of H2 (or diatomic hydrogen) contains a single bond between both the Hydrogen (H) atoms.
Let’s draw and understand this lewis dot structure in simple steps.
Steps to Draw the Lewis Structure of H2
Step #1: Calculate the total number of valence electrons
Here, the given molecule is H2 (or diatomic hydrogen). In order to draw the lewis structure of H2, first of all you have to find the total number of valence electrons present in the H2 molecule.
(Valence electrons are the number of electrons present in the outermost shell of an atom).
So, let’s calculate this first.
- For Hydrogen:
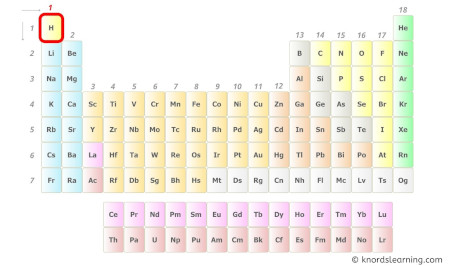
Hydrogen is a group 1 element on the periodic table. [1]
Hence, the valence electron present in hydrogen is 1 (see below image).
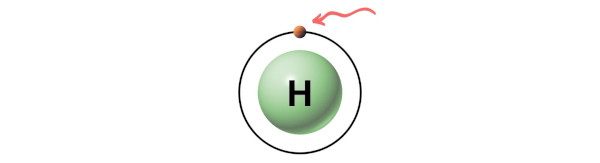
Hence in a H2 molecule,
Valence electron given by each Hydrogen (H) atom = 1
So, total number of Valence electrons in H2 molecule = 1(2) = 2
Step #2: Make the rough sketch
While selecting the atom, you have to put the least electronegative atom at the center.
But here in the H2 molecule, both the atoms are same. So you can consider any of the atoms as a center atom.

Step #3: Put two electrons between the atoms to represent a chemical bond
Now in the above sketch of H2 molecule, put the two electrons (i.e electron pair) between each hydrogen atom to represent a chemical bond between them.

These pairs of electrons present between the Hydrogen (H) atoms form a chemical bond, which bonds the hydrogen atoms with each other in a H2 molecule.
Step #4: Complete the octet (or duplet) on the atoms
Hydrogen requires only 2 electrons to have a complete outer shell.
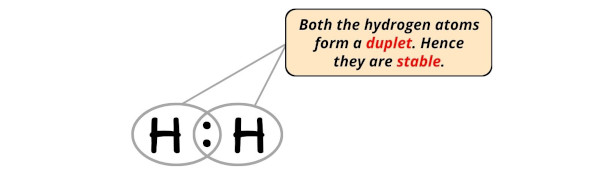
You can see in the above image that both the hydrogen atoms form a duplet.
Also, the 2 valence electrons of H2 molecule (as calculated in step #1) are used in the above structure. And hence, the above lewis structure of H2 is the final stable structure only.
The electron pair (:) in the lewis dot structure of H2 represents the single bond ( | ). So the above lewis dot structure of H2 can also be represented as shown below.
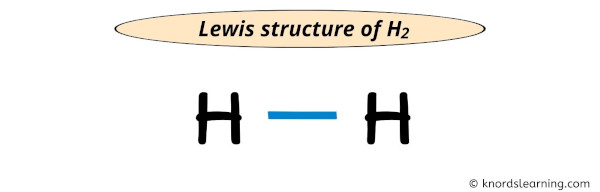
Well, this was a very simple lewis structure.
For more practice and better understanding, you can try other lewis structures listed below.
Related lewis structures for your practice:
Lewis structure of N3-
Lewis structure of BH3
Lewis structure of C2H6
Lewis structure of COCl2
Lewis structure of BrF5
Article by;
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.


sexygrannypics.com nude granny may not be as spry because they was previously, but don’t underestimate grandma’s capability to rock your world with pleasure! Despite how old they are, they emit confidence and take charge, having a great time. Sometimes they’re the people teaching younger guys something or two and loving every moment of it. Grandmas in adult enjoyment fearlessly explore their desires and beat to satisfy themselves and their companions. Some individuals might find it a bit taboo to speak about this genre. MILF porn is totally fine, but grandma porn? That’s a different story. It’s viewed as controversial and not everyone is into it. But hey, if that’s what you’re into, no judgment here! Lots of people enjoy mature adult entertainment, so it’s a popular choice for viewers.