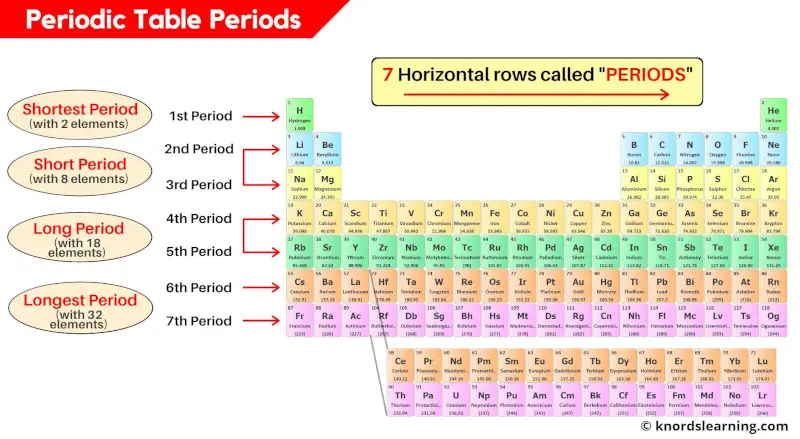
Periods in periodic table are the horizontal rows on the periodic table. There are a total 7 horizontal rows on the periodic table, that means the periodic table has 7 periods.
- Period 1: Shortest period (having 2 elements)
- Period 2 and 3: Short period (having 8 elements each)
- Period 4 and 5: Long period (having 18 elements each)
- Period 6 and 7: Longest period (having 32 elements each)
Let’s discuss each of these periods of periodic table one by one.
Period 1: Shortest period
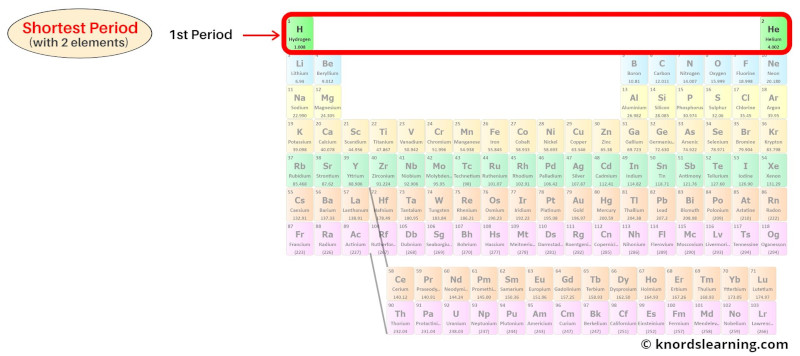
The 1st period of the periodic table is known as the shortest period.
Period 1 is the shortest period because it has only 2 elements in it.
The number of shell present in the period 1 elements is 1.
Elements of period 1 are given in the table below.
Period 1 elements:
| Group | 1 | 18 |
| Atomic no. | 1 | 2 |
| Element | H | He |
Period 2 and 3: Short period
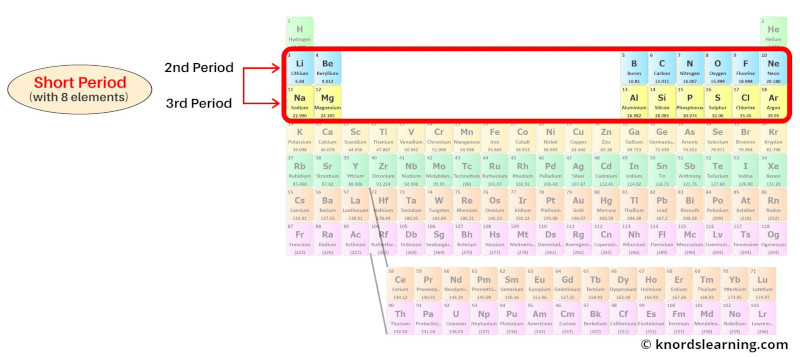
The 2nd period and the 3rd period of the periodic table are known as the short periods.
Period 2 and period 3 are the short period because they have 8 elements in it.
Period 2 elements have 2 energy shells while period 3 elements have 3 energy shells.
Elements of period 2 and period 3 are given in the tables below.
Period 2 elements:
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Atomic no. | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Element | Li | Be | B | C | N | O | Fl | Ne |
Period 3 elements:
| Group | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Atomic no. | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
Period 4 and 5: Long period
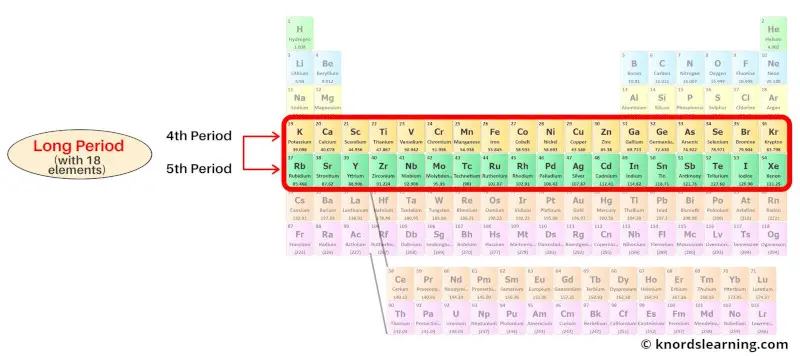
The 4th period and the 5th period of the periodic table are known as the long periods.
Period 4 and period 5 are the long period because they have 18 elements in it.
Period 4 elements have 4 energy shells while period 5 elements have 5 energy shells.
Elements of period 4 and period 5 are given in the tables below.
Period 4 elements:
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Atomic no. | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
| Element | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Sc | Br | Kr |
Period 5 elements:
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Atomic no. | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 |
| Element | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
Period 6 and 7: Longest period
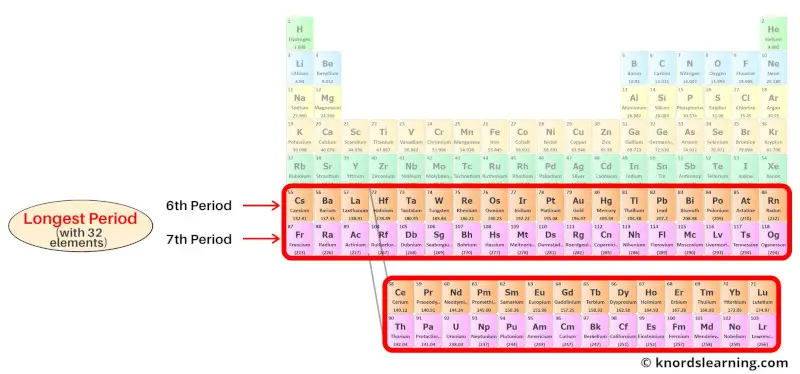
The 6th period and the 7th period of the periodic table are known as the longest periods.
Period 6 and period 7 are the longest period because they have 32 elements in it.
Period 6 elements have 6 energy shells while period 7 elements have 7 energy shells.
Elements of period 6 and period 7 are given in the tables below.
Period 6 elements:
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||
| Atomic no. | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 | 86 |
| Element | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
Period 7 elements:
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||
| Atomic no. | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 |
| Element | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
External resources:
- Period | chemistry. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/period-chemistry
- Period (periodic table) – Wikipedia. (2008, February 5). Period (Periodic Table) – Wikipedia. https://en.wikipedia.org/wiki/Period_(periodic_table)
- 6.4: Modern Periodic Table- Periods and Groups. (2016, June 24). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/06%3A_The_Periodic_Table/6.04%3A_Modern_Periodic_Table-_Periods_and_Groups
- Reading the Periodic Table. (n.d.). Reading the Periodic Table. http://www.csun.edu/~psk17793/G%20Chemistry/reading_the_periodic_table.htm
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
