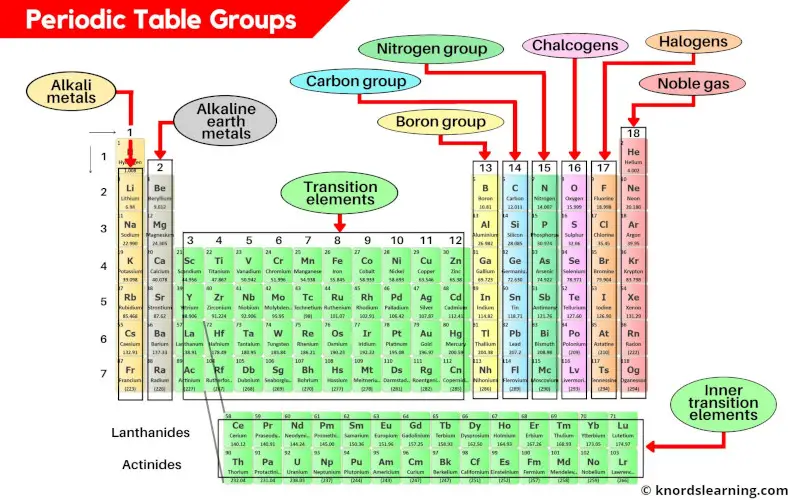
Periodic table groups are the vertical columns on the periodic table. There are a total of 18 groups (vertical columns) on the periodic table.
- Group 1: Alkali metals (hydrogen excluded)
- Group 2: Alkaline earth metals
- Group 3-11: Transition and inner transition metals group
- Group 13: Boron group
- Group 14: Carbon group
- Group 15: Nitrogen group (pnictogens)
- Group 16: Oxygen group (chalcogens)
- Group 17: Halogen group
- Group 18: Noble gases
Let’s discuss each of these groups of periodic table one by one.
Group 1: Alkali metals
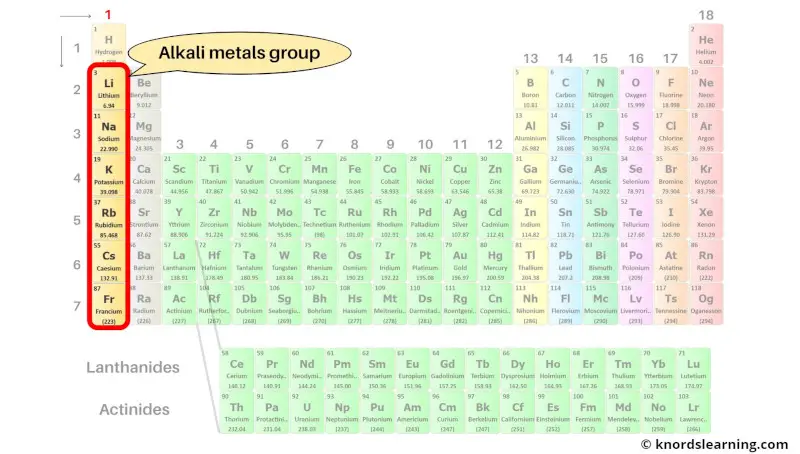
Alkali metals are the group 1 elements present on the periodic table. Hydrogen is not included in the alkali metals group because it is classified as a nonmetal.
The list of alkali metals with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 3 | Li | Lithium |
| 11 | Na | Sodium |
| 19 | K | Potassium |
| 37 | Rb | Rubidium |
| 55 | Cs | Cesium |
| 87 | Fr | Francium |
Group 2: Alkaline earth metals
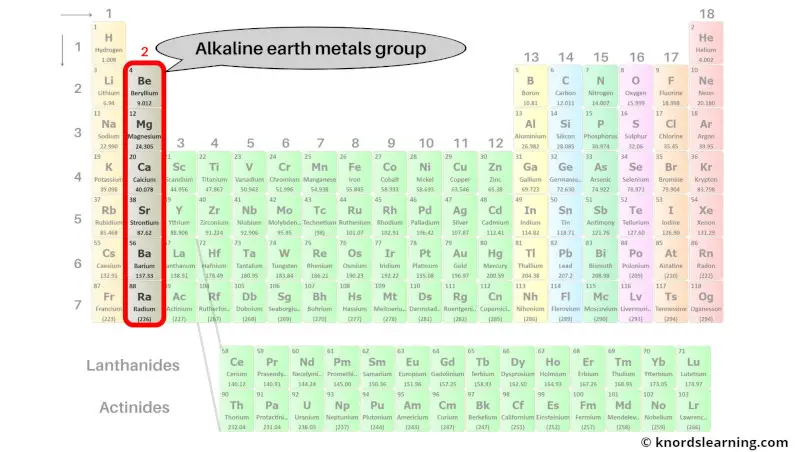
Alkaline earth metals are the group 2 elements present on the periodic table.
The list of alkaline earth metals with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 4 | Be | Beryllium |
| 12 | Mg | Magnesium |
| 20 | Ca | Calcium |
| 38 | Sr | Strontium |
| 56 | Ba | Barium |
| 88 | Ra | Radium |
Group 3-11: Transition and Inner transition metals group
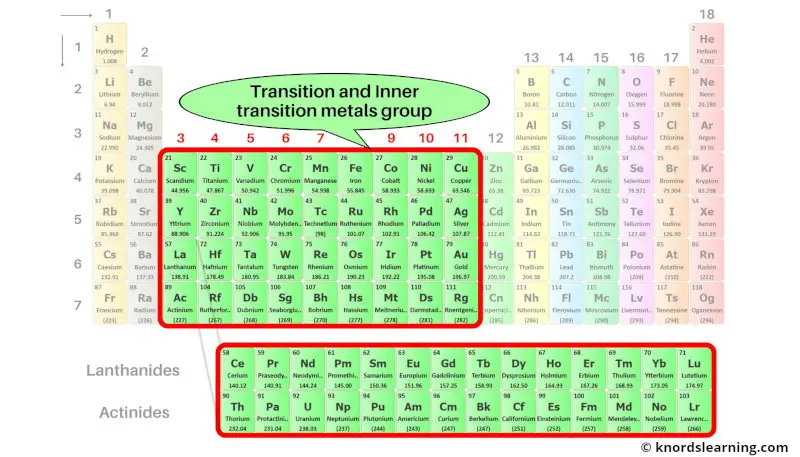
Transition metals and Inner transition metals are the elements that are lying from group 3 to group 11.
The elements lying in the two rows which are lying at the bottom of the periodic table are the inner transition metals.
These elements are the part of transition metals only, but they have different properties. So they are placed separately at the bottom of the periodic table.
These two rows of inner transition elements are known as lanthanides and actinides.
Group 13: Boron group
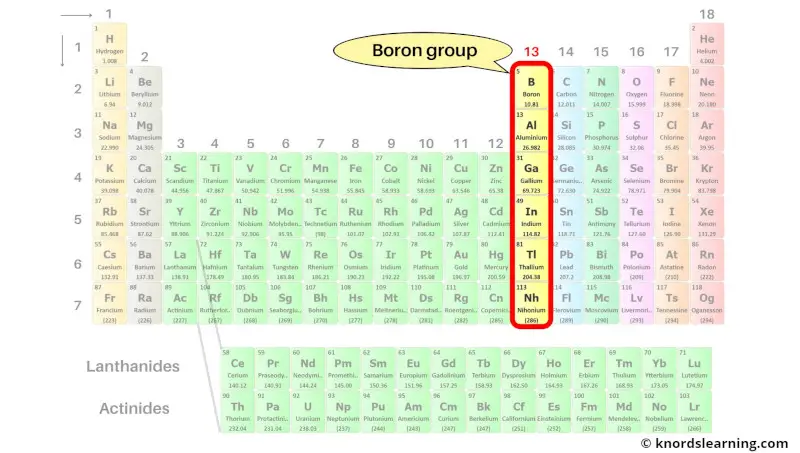
Boron group includes the group 13 elements present on the periodic table.
The list of boron group elements with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 5 | B | Boron |
| 13 | Al | Aluminum |
| 31 | Ga | Gallium |
| 49 | In | Indium |
| 81 | Tl | Thallium |
| 113 | Nh | Nihonium |
Group 14: Carbon group
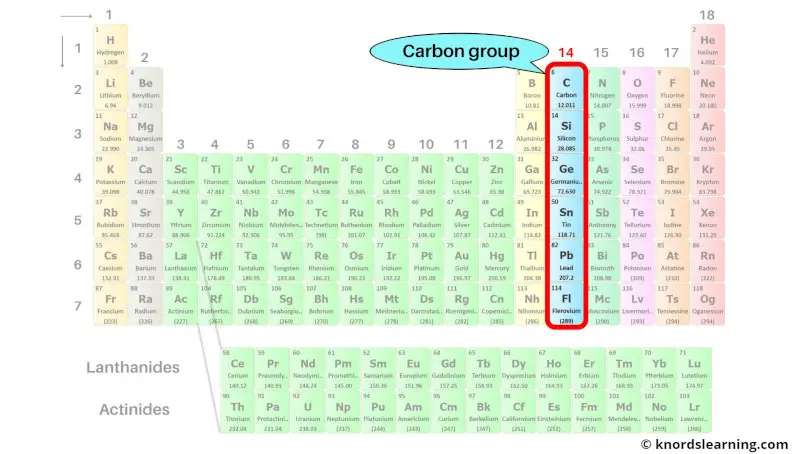
Carbon group includes the group 14 elements present on the periodic table.
The list of carbon group elements with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 6 | C | Carbon |
| 14 | Si | Silicon |
| 32 | Ge | Germanium |
| 50 | Sn | Tin |
| 82 | Pb | Lead |
| 114 | Fl | Flerovium |
Group 15: Nitrogen group (pnictogens)
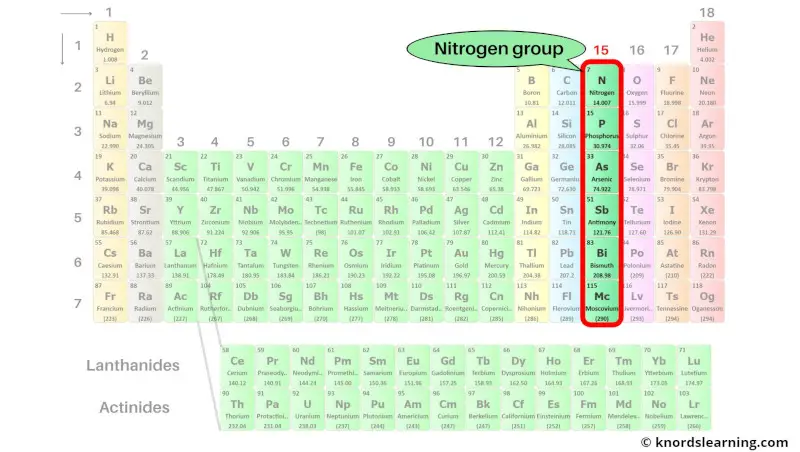
Nitrogen group (also known as pnictogens) includes the group 15 elements present on the periodic table.
The list of nitrogen group elements with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 7 | N | Nitrogen |
| 15 | P | Phosphorus |
| 33 | As | Arsenic |
| 51 | Sb | Antimony |
| 83 | Bi | Bismuth |
| 115 | Mc | Moscovium |
Group 16: Oxygen group (chalcogens)
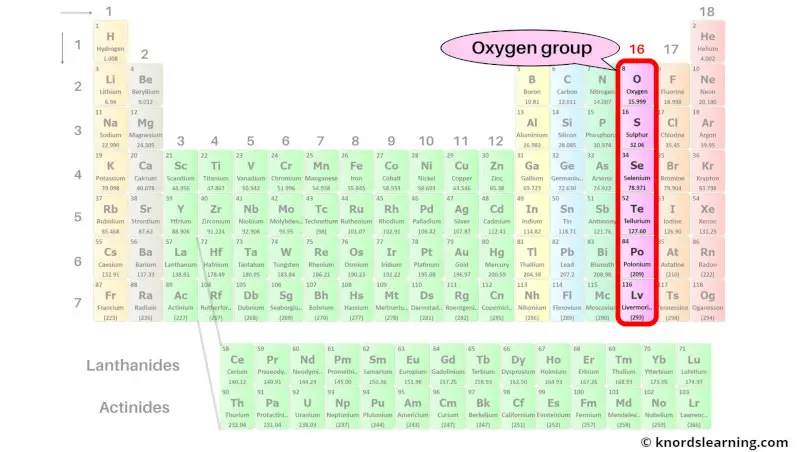
Oxygen group (also known as chalcogens) includes the group 16 elements present on the periodic table.
The list of oxygen group elements with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 8 | O | Oxygen |
| 16 | S | Sulfur |
| 34 | Se | Selenium |
| 52 | Te | Tellurium |
| 84 | Po | Polonium |
| 116 | Lv | Livermorium |
Group 17: Halogen group
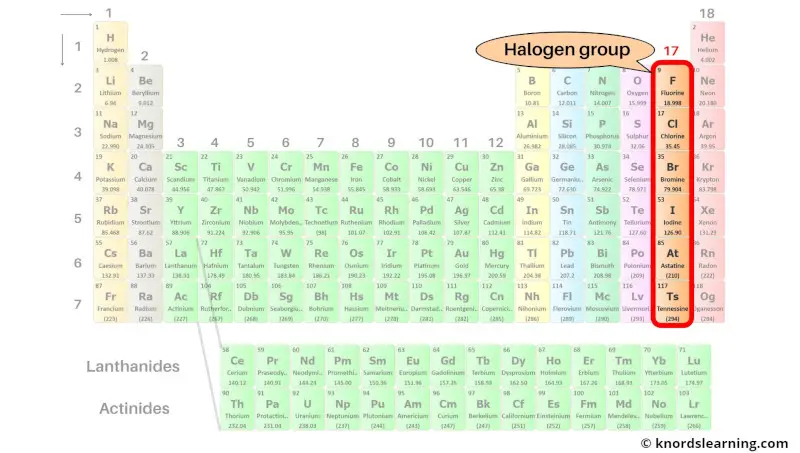
Halogen group includes the group 17 elements present on the periodic table.
The list of halogen group elements with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 9 | F | Fluorine |
| 17 | Cl | Chlorine |
| 35 | Br | Bromine |
| 53 | I | Iodine |
| 85 | At | Astatine |
| 117 | Ts | Tennessine |
Group 18: Noble gases group
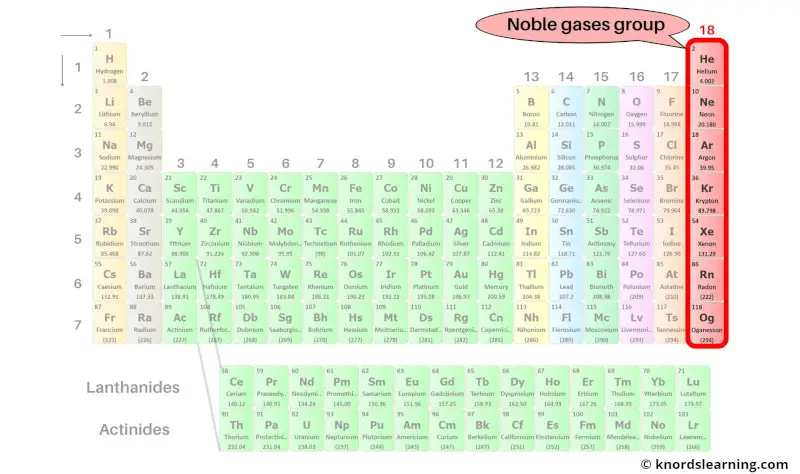
Noble gases group includes the group 18 elements present on the periodic table.
The list of noble gases with its atomic number and symbol is mentioned below.
| Atomic number | Symbol | Name of element |
| 2 | He | Helium |
| 10 | Ne | Neon |
| 18 | Ar | Argon |
| 36 | Kr | Krypton |
| 54 | Xe | Xenon |
| 86 | Rn | Radon |
| 118 | Og | Oganesson |
External resources:
- Elements Organized by Group. (2015, July 26). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Group
- Reading the Periodic Table. (n.d.). Reading the Periodic Table. http://www.csun.edu/~psk17793/G%20Chemistry/reading_the_periodic_table.htm
- Groups and periods – The periodic table – GCSE Chemistry (Single Science) Revision – WJEC – BBC Bitesize. (n.d.). BBC Bitesize. https://www.bbc.co.uk/bitesize/guides/zv2f3k7/revision/2
- IU.edu. (n.d.). https://cpanhd.sitehost.iu.edu/C101webnotes/composition/elements.html
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/group.shtml
- O. (n.d.). 2.5 The Periodic Table – Chemistry. 2.5 the Periodic Table – Chemistry. https://iu.pressbooks.pub/openstaxchemistry/chapter/2-5-the-periodic-table/
- Group (periodic table) – Wikipedia. (2018, September 15). Group (Periodic Table) – Wikipedia. https://en.wikipedia.org/wiki/Group_(periodic_table)
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

