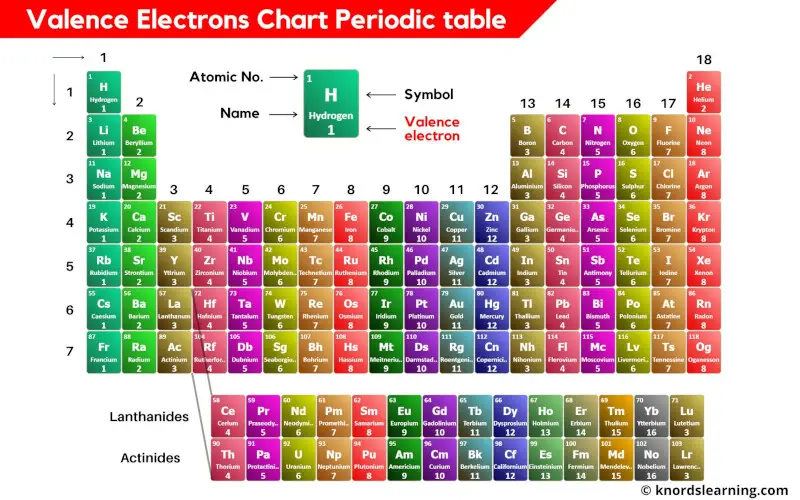
Periodic table with valence electrons is shown in the above image.
Points to remember:
- Valence electrons are the electrons present in the outermost shell of an atom.
- For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit.
- For most of the transition elements and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Valence Electrons Chart of the Elements of Periodic Table
| Atomic number | Elements | Valence electrons |
|---|---|---|
| 1 | Valence electrons in Hydrogen (H) | 1 |
| 2 | Valence electrons in Helium (He) | 2 |
| 3 | Valence electrons in Lithium (Li) | 1 |
| 4 | Valence electrons in Beryllium (Be) | 2 |
| 5 | Valence electrons in Boron (B) | 3 |
| 6 | Valence electrons in Carbon (C) | 4 |
| 7 | Valence electrons in Nitrogen (N) | 5 |
| 8 | Valence electrons in Oxygen (O) | 6 |
| 9 | Valence electrons in Fluorine (F) | 7 |
| 10 | Valence electrons in Neon (Ne) | 8 |
| 11 | Valence electrons in Sodium (Na) | 1 |
| 12 | Valence electrons in Magnesium (Mg) | 2 |
| 13 | Valence electrons in Aluminum (Al) | 3 |
| 14 | Valence electrons in Silicon (Si) | 4 |
| 15 | Valence electrons in Phosphorus (P) | 5 |
| 16 | Valence electrons in Sulfur (S) | 6 |
| 17 | Valence electrons in Chlorine (Cl) | 7 |
| 18 | Valence electrons in Argon (Ar) | 8 |
| 19 | Valence electrons in Potassium (K) | 1 |
| 20 | Valence electrons in Calcium (Ca) | 2 |
| 21 | Valence electrons in Scandium (Sc) | 3 |
| 22 | Valence electrons in Titanium (Ti) | 4 |
| 23 | Valence electrons in Vanadium (V) | 5 |
| 24 | Valence electrons in Chromium (Cr) | 6 |
| 25 | Valence electrons in Manganese (Mn) | 7 |
| 26 | Valence electrons in Iron (Fe) | 8 |
| 27 | Valence electrons in Cobalt (Co) | 9 |
| 28 | Valence electrons in Nickel (Ni) | 10 |
| 29 | Valence electrons in Copper (Cu) | 11 |
| 30 | Valence electrons in Zinc (Zn) | 12 |
| 31 | Valence electrons in Gallium (Ga) | 3 |
| 32 | Valence electrons in Germanium (Ge) | 4 |
| 33 | Valence electrons in Arsenic (As) | 5 |
| 34 | Valence electrons in Selenium (Se) | 6 |
| 35 | Valence electrons in Bromine (Br) | 7 |
| 36 | Valence electrons in Krypton (Kr) | 8 |
| 37 | Valence electrons in Rubidium (Rb) | 1 |
| 38 | Valence electrons in Strontium (Sr) | 2 |
| 39 | Valence electrons in Yttrium (Y) | 3 |
| 40 | Valence electrons in Zirconium (Zr) | 4 |
| 41 | Valence electrons in Niobium (Nb) | 5 |
| 42 | Valence electrons in Molybdenum (Mo) | 6 |
| 43 | Valence electrons in Technetium (Tc) | 7 |
| 44 | Valence electrons in Ruthenium (Ru) | 8 |
| 45 | Valence electrons in Rhodium (Rh) | 9 |
| 46 | Valence electrons in Palladium (Pd) | 10 |
| 47 | Valence electrons in Silver (Ag) | 11 |
| 48 | Valence electrons in Cadmium (Cd) | 12 |
| 49 | Valence electrons in Indium (In) | 3 |
| 50 | Valence electrons in Tin (Sn) | 4 |
| 51 | Valence electrons in Antimony (Sb) | 5 |
| 52 | Valence electrons in Tellurium (Te) | 6 |
| 53 | Valence electrons in Iodine (I) | 7 |
| 54 | Valence electrons in Xenon (Xe) | 8 |
| 55 | Valence electrons in Caesium (Cs) | 1 |
| 56 | Valence electrons in Barium (Ba) | 2 |
| 57 | Valence electrons in Lanthanum | 3 |
| 58 | Valence electrons in Cerium (Ce) | 4 |
| 59 | Valence electrons in Praseodymium (Pr) | 5 |
| 60 | Valence electrons in Neodymium (Nd) | 6 |
| 61 | Valence electrons in Promethium (Pm) | 7 |
| 62 | Valence electrons in Samarium (Sm) | 8 |
| 63 | Valence electrons in Europium (Eu) | 9 |
| 64 | Valence electrons in Gadolinium (Gd) | 10 |
| 65 | Valence electrons in Terbium (Tb) | 11 |
| 66 | Valence electrons in Dysprosium (Dy) | 12 |
| 67 | Valence electrons in Holmium (Ho) | 13 |
| 68 | Valence electrons in Erbium (Er) | 14 |
| 69 | Valence electrons in Thulium (Tm) | 15 |
| 70 | Valence electrons in Ytterbium (Yb) | 16 |
| 71 | Valence electrons in Lutetium (Lu) | 3 |
| 72 | Valence electrons in Hafnium (Hf) | 4 |
| 73 | Valence electrons in Tantalum (Ta) | 5 |
| 74 | Valence electrons in Tungsten (W) | 6 |
| 75 | Valence electrons in Rhenium (Re) | 7 |
| 76 | Valence electrons in Osmium (Os) | 8 |
| 77 | Valence electrons in Iridium (Ir) | 9 |
| 78 | Valence electrons in Platinum (Pt) | 10 |
| 79 | Valence electrons in Gold (Au) | 11 |
| 80 | Valence electrons in Mercury (Hg) | 12 |
| 81 | Valence electrons in Thallium (Tl) | 3 |
| 82 | Valence electrons in Lead (Pb) | 4 |
| 83 | Valence electrons in Bismuth (Bi) | 5 |
| 84 | Valence electrons in Polonium (Po) | 6 |
| 85 | Valence electrons in Astatine (At) | 7 |
| 86 | Valence electrons in Radon (Rn) | 8 |
| 87 | Valence electrons in Francium (Fr) | 1 |
| 88 | Valence electrons in Radium (Ra) | 2 |
| 89 | Valence electrons in Actinium (Ac) | 3 |
| 90 | Valence electrons in Thorium (Th) | 4 |
| 91 | Valence electrons in Protactinium (Pa) | 5 |
| 92 | Valence electrons in Uranium (U) | 6 |
| 93 | Valence electrons in Neptunium (Np) | 7 |
| 94 | Valence electrons in Plutonium (Pu) | 8 |
| 95 | Valence electrons in Americium (Am) | 9 |
| 96 | Valence electrons in Curium (Cm) | 10 |
| 97 | Valence electrons in Berkelium (Bk) | 11 |
| 98 | Valence electrons in Californium (Cf) | 12 |
| 99 | Valence electrons in Einsteinium (Es) | 13 |
| 100 | Valence electrons in Fermium (Fm) | 14 |
| 101 | Valence electrons in Mendelevium (Md) | 15 |
| 102 | Valence electrons in Nobelium (No) | 16 |
| 103 | Valence electrons in Lawrencium (Lr) | 3 |
| 104 | Valence electrons in Rutherfordium (Rf) | 4 |
| 105 | Valence electrons in Dubnium (Db) | 5 |
| 106 | Valence electrons in Seaborgium (Sg) | 6 |
| 107 | Valence electrons in Bohrium (Bh) | 7 |
| 108 | Valence electrons in Hassium (Hs) | 8 |
| 109 | Valence electrons in Meitnerium (Mt) | 9 |
| 110 | Valence electrons in Darmstadtium (Ds) | 10 |
| 111 | Valence electrons in Roentgenium (Rg) | 11 |
| 112 | Valence electrons in Copernicium (Cn) | 12 |
| 113 | Valence electrons in Nihonium (Nh) | 3 |
| 114 | Valence electrons in Flerovium (Fl) | 4 |
| 115 | Valence electrons in Moscovium (Mc) | 5 |
| 116 | Valence electrons in Livermorium (Lv) | 6 |
| 117 | Valence electrons in Tennessine (Ts) | 7 |
| 118 | Valence electrons in Oganesson (Og) | 8 |
External resources:
- Valence Electrons. (n.d.). Valence Electrons. https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/index.php
- Valence electron – Wikipedia. (2022, December 28). Valence Electron – Wikipedia. https://en.wikipedia.org/wiki/Valence_electron
- Illustrated Glossary of Organic Chemistry – Valence electron. (n.d.). Illustrated Glossary of Organic Chemistry – Valence Electron. http://www.chem.ucla.edu/~harding/IGOC/V/valence_electron.html
- 1.3: Valence electrons and open valences. (2014, July 31). Chemistry LibreTexts. https://chem.libretexts.org/Courses/Purdue/Purdue%3A_Chem_26505%3A_Organic_Chemistry_I_(Lipton)/Chapter_1._Electronic_Structure_and_Chemical_Bonding/1.03_Valence_electrons_and_open_valences
Author
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
