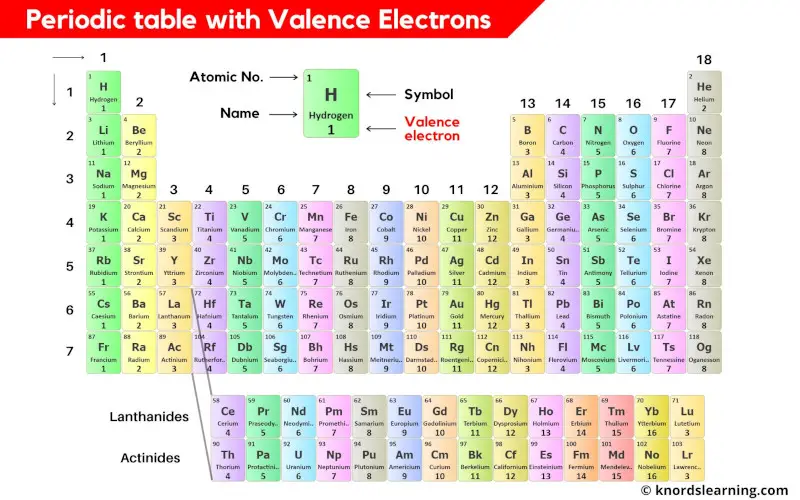
This is a periodic table with valence electrons labeled on it.
You can also download the HD image of the periodic table with valence electrons.
(The download link is given at the end of this article.)
But before that, you need to know a few things about the valence electrons of main group elements as well as valence electrons of transition and inner transition elements.
Let’s dive right into it.
Table of contents
- What are valence electrons?
- Valence electrons of transition and inner transition elements
- Periodic tables with valence electrons (labeled images)
What are valence electrons?
Valence electrons are the electrons present in the outermost orbit of an atom.
For example;
Consider the magnesium element.
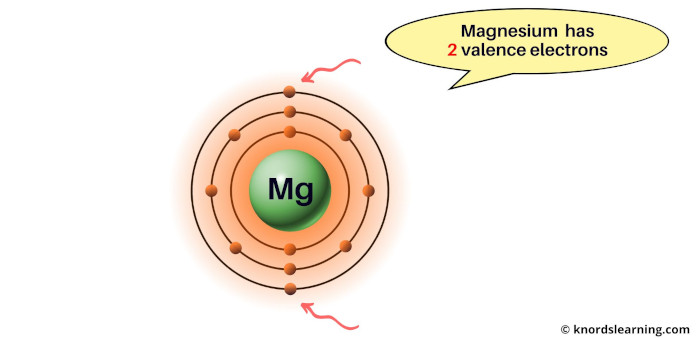
You can see that the magnesium atom has 2 electrons in its outermost orbit.
So the valence electrons of magnesium are 2.
You can also find the valence electrons by using the electron configuration of elements.
In the electron configuration, you have to see the total number of electrons present in the shell with a highest principal quantum number.
Let’s consider the example of magnesium only.
The electron configuration of magnesium is 1s2 2s2 2p6 3s2.
In this electron configuration, you can see that the highest principal quantum number is 3. And the number of electrons present in this principal quantum number is 2.
So, magnesium has 2 valence electrons.
Now, this method does not suit for transition and inner transition elements.
So let’s discuss this in short.
Valence electrons of Transition and Inner transition elements
It seems a little difficult to find the valence electrons of transition elements and inner transition elements.
About transition elements:
The transition elements have incomplete d-orbitals and the d-orbitals are very close to the outer s-orbitals.
So the electrons present in the d-orbitals as well as s-orbitals act like valence electrons.
Thus the transition elements have valence electrons ranging from 3 to 12 (See above image of periodic table).
About inner transition elements:
The inner transition elements also have a similar case.
The inner transition elements have incomplete f-orbitals and the f-orbitals are very close to outer s-orbitals.
So the electrons present in the f-orbitals as well as s-orbitals act like valence electrons.
Thus the inner transition elements have valence electrons ranging from 3 to 16 (See above image of periodic table).
Point to Remember: Mostly for transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Periodic tables with valence electrons (labeled images)
Dark color
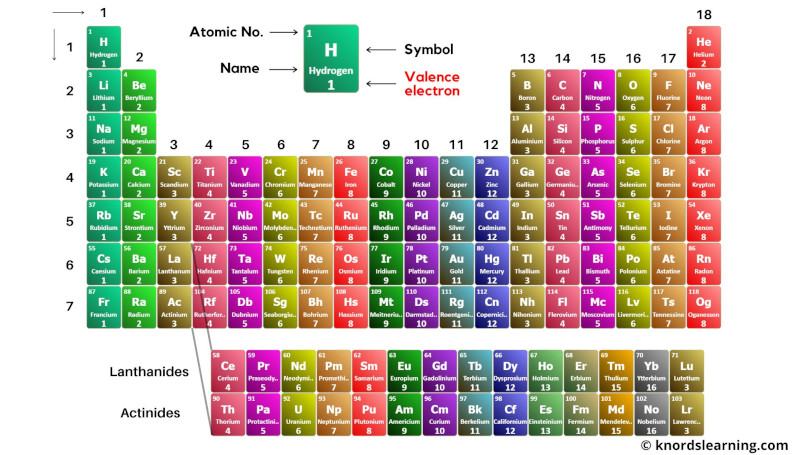
Block wise elements
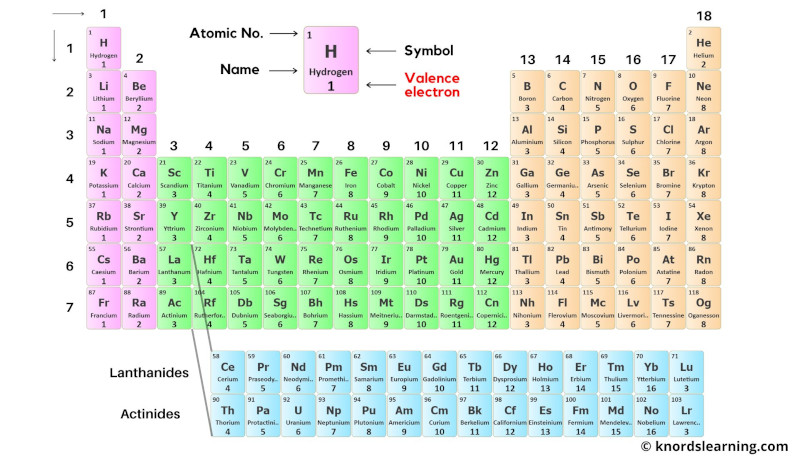
Black and white

Blue colored
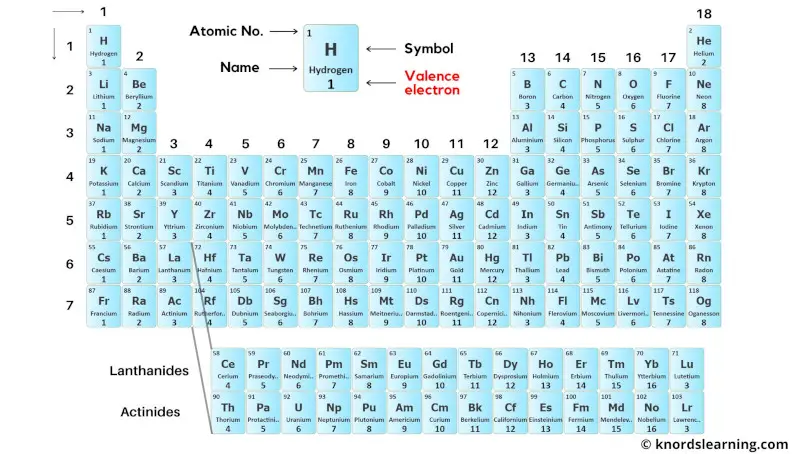
To see the valence electrons of all the elements in table form, visit this article: Valence electrons table of all the elements
Don’t forget to download the HD image of the periodic table with valence electrons from below.
External resources:
- Valence Electrons. (n.d.). Valence Electrons. https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/index.php
- Valence electron – Wikipedia. (2022, December 28). Valence Electron – Wikipedia. https://en.wikipedia.org/wiki/Valence_electron
- Illustrated Glossary of Organic Chemistry – Valence electron. (n.d.). Illustrated Glossary of Organic Chemistry – Valence Electron. http://www.chem.ucla.edu/~harding/IGOC/V/valence_electron.html
- 1.3: Valence electrons and open valences. (2014, July 31). Chemistry LibreTexts. https://chem.libretexts.org/Courses/Purdue/Purdue%3A_Chem_26505%3A_Organic_Chemistry_I_(Lipton)/Chapter_1._Electronic_Structure_and_Chemical_Bonding/1.03_Valence_electrons_and_open_valences
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

