Ready to learn how to draw the lewis structure of Acetone (C3H6O)?
Awesome!
Here, I have explained simple steps to draw the lewis dot structure of Acetone (along with images).
So let’s dive right into it!
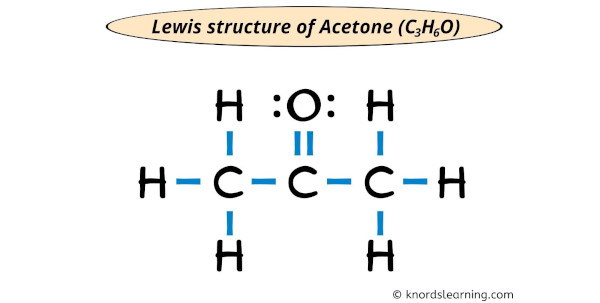
Lewis structure of Acetone (also known as Propanone or C3H6O) contains three Carbon atoms (C) in a row which have an Oxygen atom (O) attached to a central Carbon atom (C) forming a double bond. The outer Carbon atoms (C) are surrounded by the Hydrogen atoms (H). The Oxygen atom has 2 lone pairs.
Let’s draw and understand this lewis dot structure in simple steps.
3 Steps to Draw the Lewis Structure of Acetone (C3H6O)
Step #1: Calculate the total number of valence electrons
Here, the given molecule is Acetone (which has a chemical formula C3H6O). In order to draw the lewis structure of C3H6O, first of all you have to find the total number of valence electrons present in the C3H6O molecule.
(Valence electrons are the number of electrons present in the outermost shell of an atom).
So, let’s calculate this first.
- For Carbon:
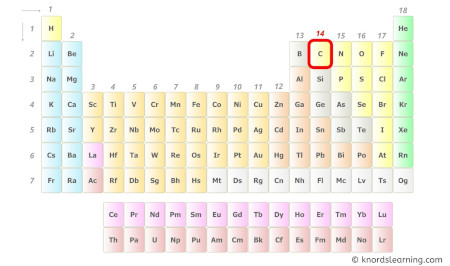
Carbon is a group 14 element on the periodic table. [1]
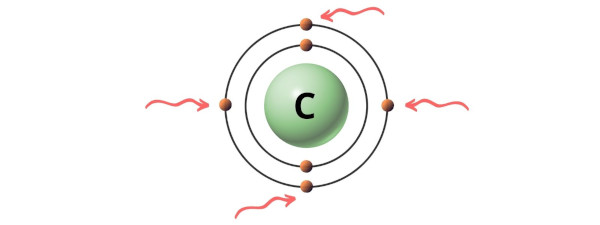
Hence, the valence electrons present in carbon is 4 (see below image).
- For Hydrogen:
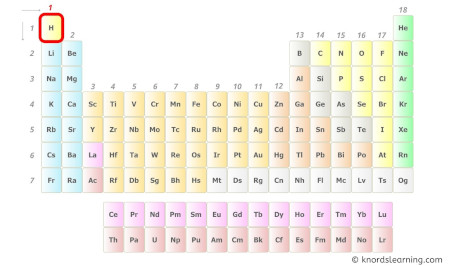
Hydrogen is a group 1 element on the periodic table. [2]
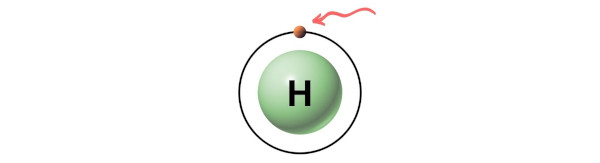
Hence, the valence electron present in hydrogen is 1 (see below image).
- For Oxygen:
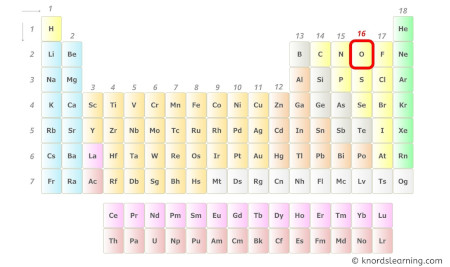
Oxygen is a group 16 element on the periodic table. [3]
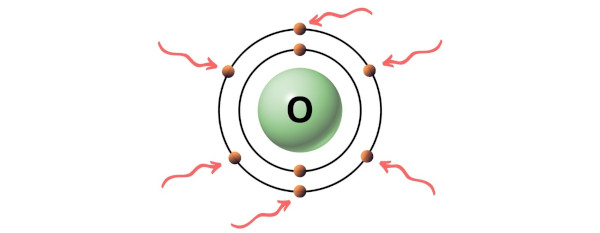
Hence, the valence electron present in oxygen is 6 (see below image).
Hence in a C3H6O molecule,
Valence electrons given by each Carbon (C) atom = 4
Valence electron given by each Hydrogen (H) atom = 1
Valence electrons given by Oxygen (O) atom = 6
So, total number of Valence electrons in C3H6O = 4(3) + 1(6) + 6 = 24
Step #2: Make the rough sketch
Acetone is a simple ketone that has 3 carbon atoms in a row and the central carbon atom is having an oxygen atom that is double bonded with it.
The outer carbon atoms are surrounded by hydrogen atoms.
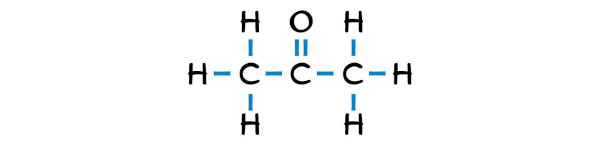
Step #3: Complete the octet (or duplet) on the outer atoms
In the Lewis structure of C3H6O, the outer atoms are hydrogen atoms as well as oxygen atom.
Hydrogen already has a duplet (see below image).
So now, you have to complete the octet on oxygen atom (because oxygen requires 8 electrons to have a complete outer shell).
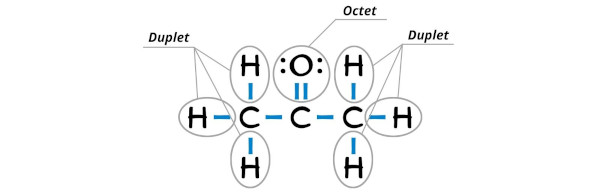
You can see in the above image that the oxygen atom forms an octet.
Also, all the 24 valence electrons of C3H6O (as calculated in step #1) are used in the above structure. And hence, the above lewis structure of C3H6O (i.e acetone) is the final stable structure only.
Well, this was a very simple lewis structure.
For more practice and better understanding, you can try other lewis structures listed below.
Related lewis structures for your practice:
Lewis Structure of POCl3
Lewis Structure of HNO2
Lewis Structure of HCO3-
Lewis Structure of C3H8 (Propane)
Lewis Structure of CH3CN
Article by;
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.