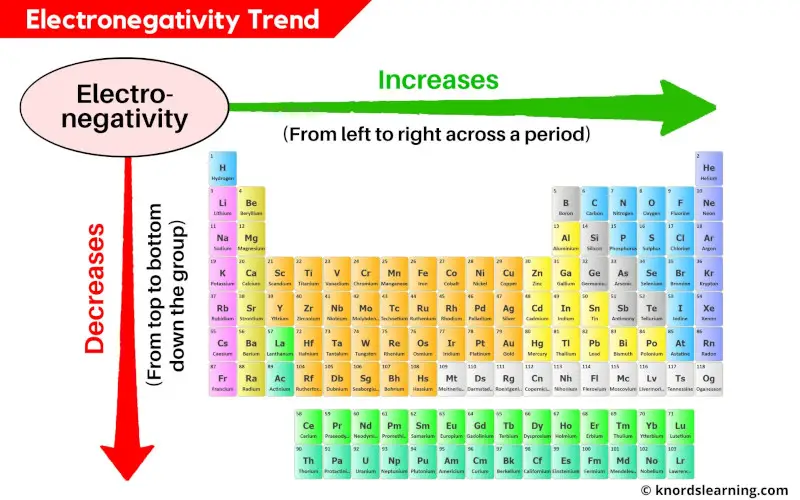
Electronegativity Trend:
Across the period (from left to right): Increases
Down the group (from top to bottom): Decreases
The electronegativity of elements increases as we move across a period (from left to right) and it decreases as we move down in a group (from top to bottom).
Well this was just an introduction about the electronegativity trends in periodic table.
But you need to understand why the electronegativity trends of elements increase across a period and why it decreases down the group.
So let’s dive right into it!
Explanation about Electronegativity Trends
Before understanding the electronegativity trends, you should know what electronegativity is?
Electronegativity is the ability of an atom to attract the shared pair of electrons.
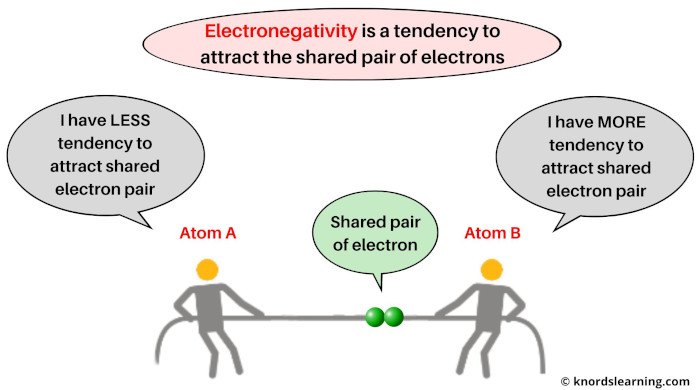
Let’s understand the concept of electronegativity with an example.
Let’s say you have two atoms, Atom A & Atom B. And they are sharing one electron pair during their chemical reaction.
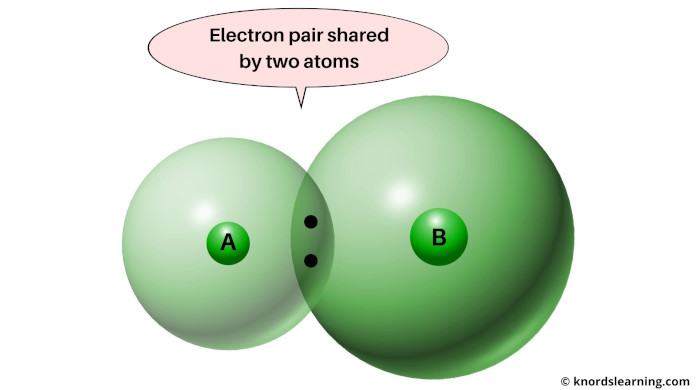
So here they are, you can see Atom A and Atom B sharing one electron pair.
Now what will happen here?
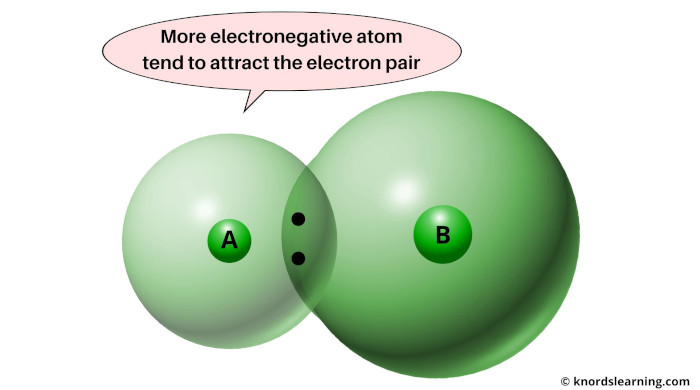
The electron pair will remain attracted towards the atom that is more electronegative.
So this phenomenon in which the electron pair remains attracted towards the more electronegative element is called electronegativity.
Now let’s understand why this happens?
The nucleus contains protons which are positively charged particles. And the shared pair of electrons has a negative charge.
So there occurs an attraction between the positively charged protons and negatively charged electron pair.
Now if the size of the atom is less, then the nucleus will exert more force of attraction on the electron pair.
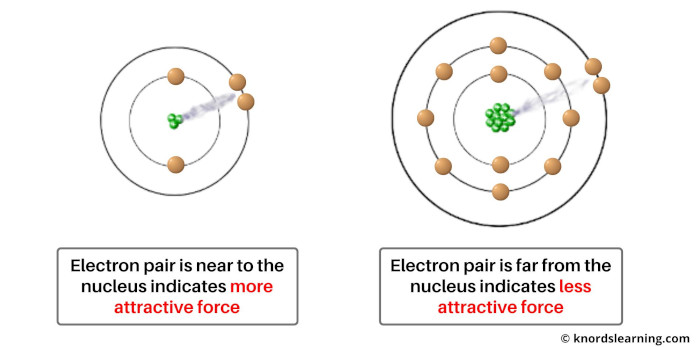
And similarly if the size of an atom is large, then the nucleus will exert less force of attraction on the electron pair.
Now if there is more attractive force, then the electron pair remains closer towards the atom.
And if there is less attractive force, then the electron pair will not remain closer towards the atom.
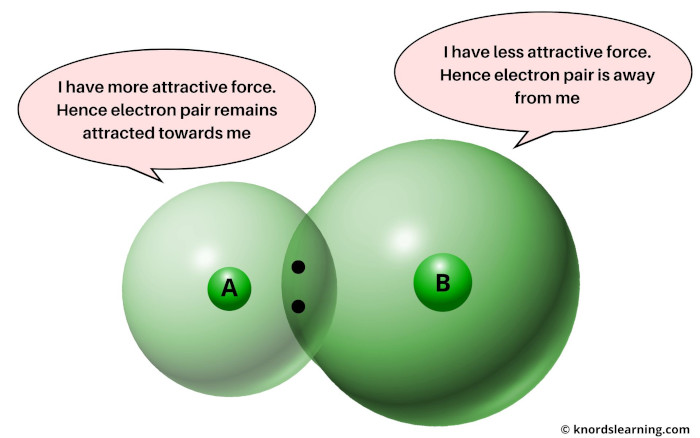
In other words, if the atomic size is less, then it will attract the electron pair and this is termed as Electronegativity.
(In other words, less the size of an atom, more is the attractive force towards the electron pair, and hence the more Electronegativity.
Or
More the size of atom, lesser the attractive force towards the electron pair, and hence the lesser Electronegativity)
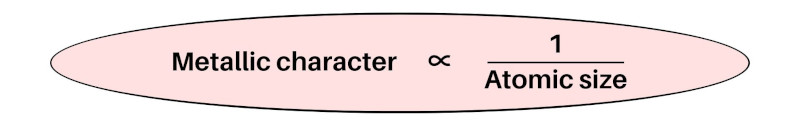
So, as the Atomic size decreases, the Electronegativity increases.
Now, you might be knowing about the atomic size trends on periodic table.
As we move across the period (from left to right), the atomic size decreases, so the electronegativity increases.
And similarly as we move down the group (from top to bottom), the atomic size increases, so the electronegativity decreases.
I hope you have understood the reason behind the electronegativity trends on the periodic table.
Checkout other trends in periodic table:
- Atomic radius trends
- Electron affinity trends
- Ionization energy trends
- Metallic character trends
- Non metallic character trends
External resources:
- McCord, P. (n.d.). Periodic Table Trends. Periodic Table Trends. https://mccord.cm.utexas.edu/chembook/page.php?chnum=3§=10
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/trends_summary.htm
- Periodic Table: Trends. (n.d.). Periodic Table: Trends. https://www.rsc.org/periodic-table/trends
- Periodic trends – Wikipedia. (2022, July 2). Periodic Trends – Wikipedia. https://en.wikipedia.org/wiki/Periodic_trends
- Periodic Trends. (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
- University of Alabama in Huntsville. https://www.uah.edu/images/administrative/student-success-center/resources/handouts/handouts_2019/periodic_trends.pdf
- Americal Chemical Society. https://www.acs.org/content/dam/acsorg/education/students/highschool/chemistryclubs/infographics/mastering-periodic-trends-infographic.pdf
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.