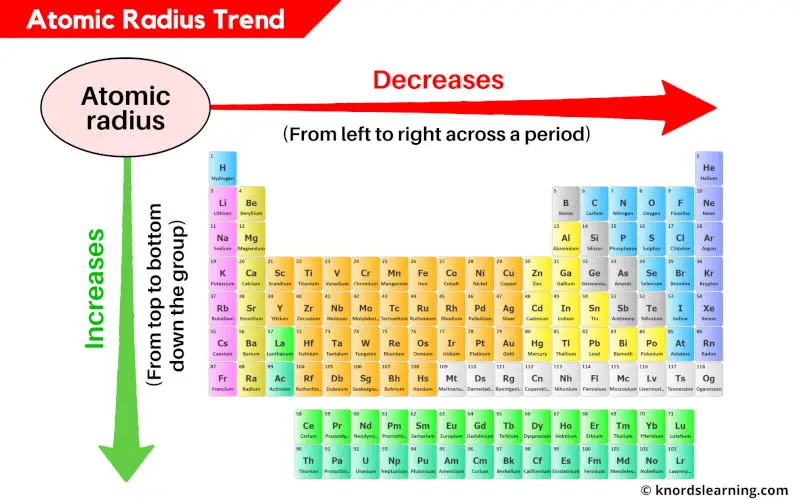
Atomic Radius Trend:
Across the period (from left to right): Decreases
Down the group (from top to bottom): Increases
The atomic radius (or atomic size) of the elements decreases as we move across a period (from left to right) and it increases as we move down in a group (from top to bottom).
Well this was just an introduction about the atomic radius trends (or atomic size trends) in periodic table.
But you need to understand why the atomic radius of elements decreases across a period and why it increases down the group.
So let’s dive right into it!
Explanation about Atomic Radius Trends
You know that the elements are arranged in the periodic table on the basis of increasing order of their atomic number.
That means when we move from left to right in the periodic table, the atomic number of elements increases.
As the atomic number increases, the number of protons inside the nucleus of an atom increases.
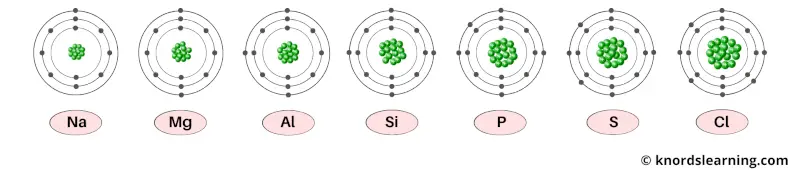
The protons are positively charged particles. So more the number of protons, more is the positive charge present in the nucleus.
The electrons which revolve around the nucleus are negatively charged particles.
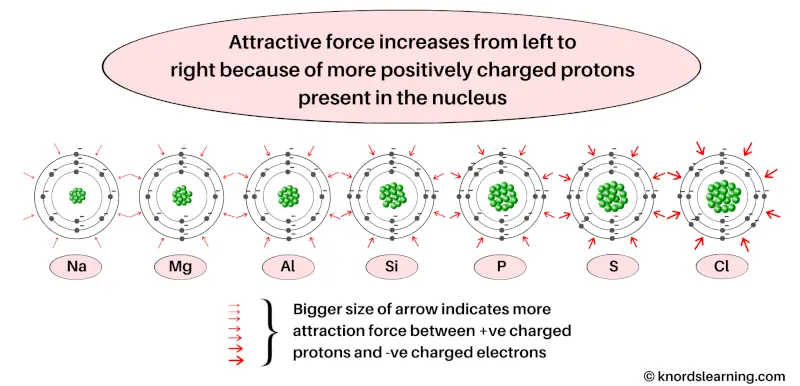
As the positive charge of the nucleus increases from left to right, it attracts the negatively charged electrons with a greater force.
Also the elements lying in the same period have the same number of orbits.
That means the distance of the electrons from the nucleus is also same (for the elements lying in the same period).
So, because of the more attractive force of nucleus and the same number of orbits across a period, the size of atom shrinks.
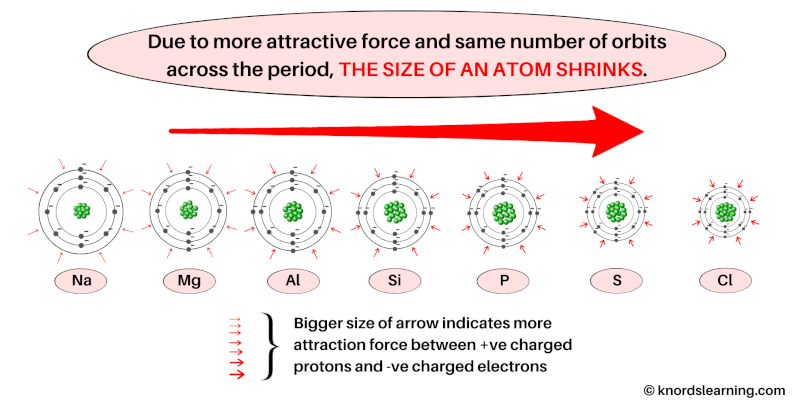
You can see in the above image that, for the elements of the same period, the atomic size shrinks as we move from left to right.
Now let’s see why the atomic radius increases as we move down the group (from top to bottom).
As we move from top to bottom in a group, the number of orbits of the elements increases.
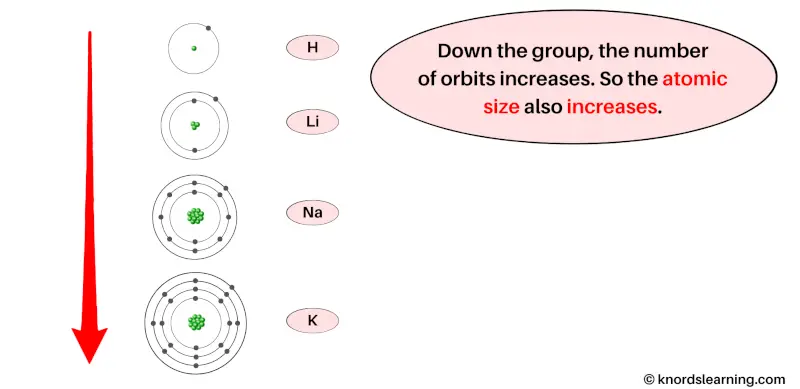
You can see in the above image that for the elements of the same group, the number of orbits increases as we move from top to bottom in a group.
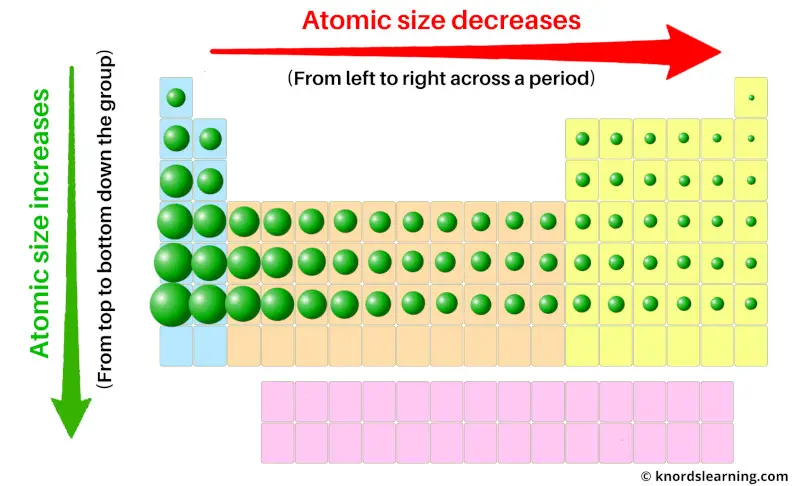
Thus the atomic radius (or atomic size) of the elements increases down the group (from top to bottom).
I hope you have understood the reason behind the atomic radius trends on the periodic table.
Checkout other trends in periodic table:
- Electronegativity trends
- Electron affinity trends
- Ionization energy trends
- Metallic character trends
- Non metallic character trends
External resources:
- McCord, P. (n.d.). Periodic Table Trends. Periodic Table Trends. https://mccord.cm.utexas.edu/chembook/page.php?chnum=3§=10
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/trends_summary.htm
- Periodic Table: Trends. (n.d.). Periodic Table: Trends. https://www.rsc.org/periodic-table/trends
- Periodic trends – Wikipedia. (2022, July 2). Periodic Trends – Wikipedia. https://en.wikipedia.org/wiki/Periodic_trends
- Periodic Trends. (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
- University of Alabama in Huntsville. https://www.uah.edu/images/administrative/student-success-center/resources/handouts/handouts_2019/periodic_trends.pdf
- Americal Chemical Society. https://www.acs.org/content/dam/acsorg/education/students/highschool/chemistryclubs/infographics/mastering-periodic-trends-infographic.pdf
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

