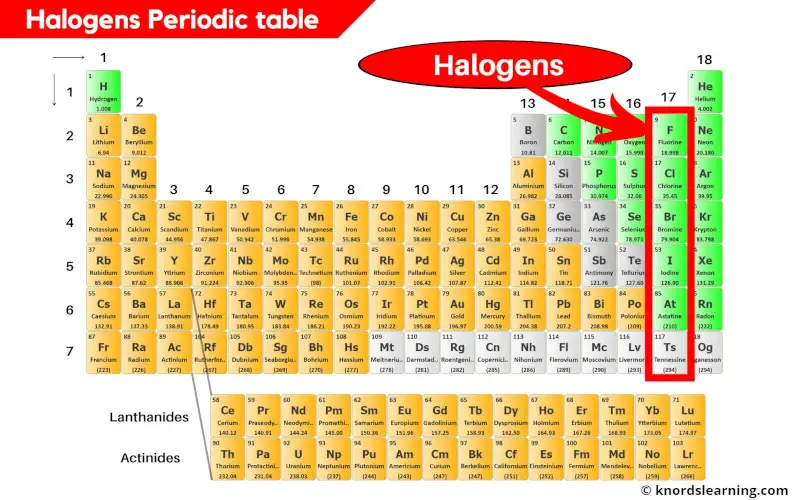
Halogens are the reactive nonmetallic elements that are found in the group 17 of the periodic table.
There are 5 or 6 halogens present on the periodic table which are listed below.
- Fluorine (F)
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
- Astatine (At) and
- Tennessine (Ts) [if included]
Well there are many more things to know about halogens of the periodic table.
So let’s dive right into it.
Table of contents
- What are Halogens?
- Most reactive Halogen on the periodic table
- List of Halogens
- Is Astatine a halogen?
- Physical properties of Halogens
- Chemical properties of Halogens
What are Halogens?
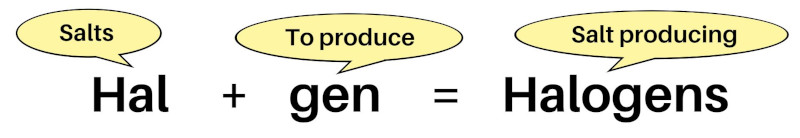
The word halogen is made of two greek words;
- Hal and
- Gen
The “Hal” means “salts”, and “Gen” means “to produce”. [1]
So, Halogens means “salt producing elements”.
Halogens produce salts when they chemically react with metals.
Let me give you a simple example of salt that is produced by reaction of halogen with metal.
2Na + Cl2 ———> 2NaCl
In the above chemical reaction, Sodium (Na) is a metal and Chlorine (Cl) is a halogen.
So when they chemically react with each other, a salt (i.e sodium chloride (NaCl)) is formed.
All the group 17 elements have the same chemical properties of forming salts on reacting with metals.
Hence the elements present in group 17 are known as salt producing elements (i.e halogens).
Most reactive Halogen on the periodic table
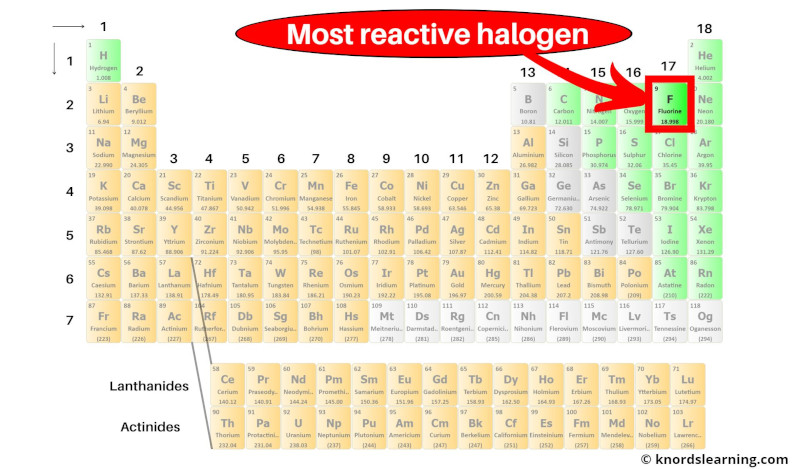
Fluorine is the most reactive halogen (and also a most reactive nonmetal) on the periodic table.
But what is the reason behind high reactivity of fluorine?
Actually fluorine is a nonmetal and it has 7 valence electrons.
Now it requires only 1 more electron to form a stable octet (i.e 7 + 1 = 8).
Also, fluorine has a very small atomic radius.
Because of the smaller atomic radius, fluorine has higher electronegativity (in simple words, the nucleus of fluorine can easily attract the electron from the other element during a chemical reaction.)
And as fluorine has a higher tendency to attract the electron from the other atom, it is a highly reactive halogen element.
List of Halogens
The list of halogens present of the periodic table is mentioned below.
| Atomic number | Name and symbol of elements |
|---|---|
| 9 | Fluorine (F) |
| 17 | Chlorine (Cl) |
| 35 | Bromine (Br) |
| 53 | Iodine (I) |
| 85 | Astatine (At) |
Is Astatine a halogen?
Astatine may be a halogen or it may not be a halogen.
Reason?
I’ll explain to you with some proof.
First of all, Astatine is a radioactive element which is made in a laboratory.
And it has a very short half life. The longest half life of a stable isotope Astatine-210 is 8.1 hours and of Astatine-211 is 7.2 hours.
That means half of the Astatine element will decay in just a few hours, which produces heat. And that is why it is very difficult to conduct experiments on it.
The only way to conduct the experiment is to do so in either gaseous phase or in the dilute solution which absorbs the heat.
But the experiments conducted using such a small quantity of elements may not give satisfactory results.
These experiments concluded that Astatine forms HAt, various salts like LiAt, interhalogens like AtI, and other halogen like bonds. (You can get this proof of experiments from google books here)
All these experiments clearly indicate that Astatine shows behavior similar to that of halogens.
But unfortunately, this is only partially true.
Because it has been found that astatine also forms At+ ions in an acidic solution.
Some researchers have found that pure astatine shows metallic characteristics in condensed phase. (Here is a research work by researchers: condensed astatine)
Also according to Cotton and Wilkinson, astatine shows more characteristics of halogens.
Hence on a newly published Periodic table, astatine is classified as a halogen.
(Note: Astatine also shows some metallic characteristics. If any authoritative sources are found further, then this article will be updated)
Physical properties of Halogens
Physical properties of halogens are mentioned below.
- Halogens are poor conductors of heat and electricity.
- Elements of the halogen group exist in all three phases (solid, liquid and gas).
- The atomic radius of halogens increases down the group in a periodic table.
- The melting point and boiling points of halogens increases down the group in a periodic table.
- The density of halogens also increases down the group.
Chemical properties of Halogens
Chemical properties of halogens are mentioned below.
- Halogens are the nonmetals that are very reactive (fluorine is the most reactive halogen.)
- Fluorine is so highly reactive that it can also form compounds with noble gasses like xenon (for example XeF4, XeF6, etc).
- During a chemical reaction, halogens gain 1 electron to form a stable octet.
- Halogens are poisonous elements.
- Halogen elements exist as diatomic molecules.
External resources:
- The Chemistry of the Halogens. (n.d.). The Chemistry of the Halogens. http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/group7.php
- Halogen – Wikipedia. (2022, March 21). Halogen – Wikipedia. https://en.wikipedia.org/wiki/Halogen
- Group 17: The Halogens. (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_17%3A_The_Halogens
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
