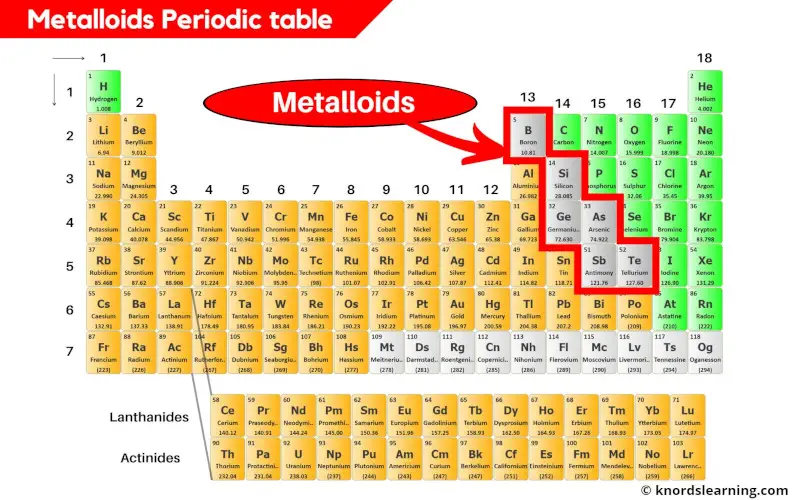
Metalloids are the elements on a periodic table that have properties that are intermediate between those of metals and nonmetals.
The metalloids are located between the metals and nonmetals on the periodic table.
Metalloids form a zigzag line between the metals and nonmetals.
There are 6 commonly known metalloids on the periodic table.
- Boron
- Silicon
- Germanium
- Arsenic
- Antimony and
- Tellurium
Well, there are a lot more things to know about metalloids.
So let’s dive right into it.
Table of contents
- What exactly are the Metalloids?
- How many Metalloids are on Periodic table?
- List of Metalloids
- Physical properties of Metalloids
- Chemical properties of Metalloids
What are Metalloids?
Metalloids are the elements that show few characteristics of metals and few characteristics of nonmetals.
For example;
Metalloids have a lustrous surface like metals, which is a property of metals. [1]
Metalloids are brittle in nature, which is a property of nonmetals. [2]
In this way, metalloids show properties of both metals and nonmetals.
In other words, we can also say that metalloids are neither completely metals nor completely nonmetals.
How many Metalloids are on the Periodic table?
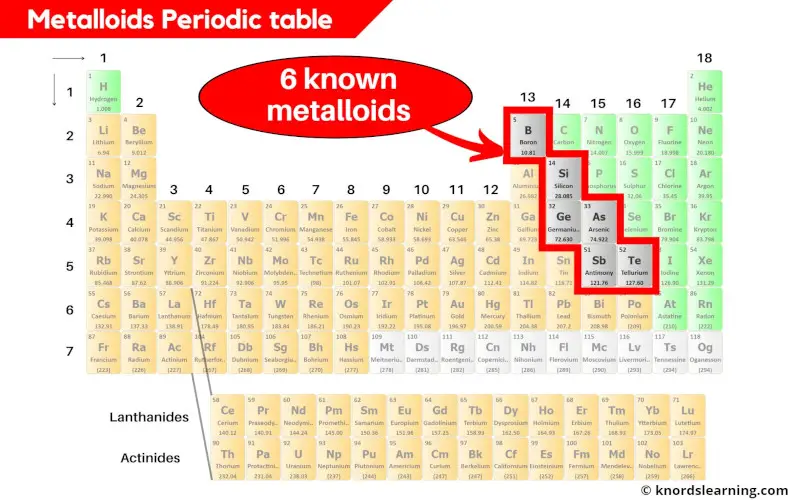
There are 6 commonly known metalloids on the periodic table.
But the element 84 and 85 (i.e Polonium (Po) and Astatine (At) respectively) also show few characteristics of metalloids.
So now you will have a question;
Is Polonium a metalloid? Is Astatine a metalloid?
As we discussed earlier, polonium and astatine show characteristics of both metals as well as nonmetals.
But according to researchers, the Polonium metals show more characteristics like that of metals and Astatine shows more characteristics like that of nonmetals (especially astatine shows characteristics like that of halogens.)
Thus the polonium and astatine are not categorized as a metalloid on the periodic table. [3]
One thing you should know about polonium and astatine is that they are synthetic elements and are prepared in the laboratory. And they have very short half-life.
Also there is no universally accepted definition of metals, nonmetals and metalloids.
That means in metallurgy the definition of metals is based on the density. In physics, the definition is based on physical properties of metals. And in chemistry, the definition is based on chemical properties.
Hence the total 6 known metalloids are shown in the above periodic table.
List of Metalloids
The list of 6 known metalloids are mentioned below.
| Atomic number | Name and Symbol of element |
|---|---|
| 5 | Boron (B) |
| 14 | Silicon (Si) |
| 32 | Germanium (Ge) |
| 33 | Arsenic (As) |
| 51 | Antimony (Sb) |
| 52 | Tellurium (Te) |
Now let’s see the physical and chemical properties of metalloids.
Physical properties of Metalloids
Most of the physical properties of metalloids are similar to that of metals.
- Metalloids are solid in state at room temperature.
- Metalloids are brittle in nature.
- Metalloids look like metals.
- The conductivity of metalloids is less than metals and more than nonmetals.
Chemical properties of Metalloids
Most of the chemical properties of metalloids are similar to that of metals.
- Metalloids are mixed with metals to form alloys which have improved properties.
- Metalloids possess different metallic allotropes as well as nonmetallic allotropes.
- Metalloids form glasses on oxidation. So they are used in making glasses.
External resources:
- Foundation, C. (n.d.). CK12-Foundation. CK12-Foundation. https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/4.5/primary/lesson/metalloids-ms-ps
- Metalloid – Wikipedia. (n.d.). Metalloid – Wikipedia. https://en.wikipedia.org/wiki/Metalloid
- Metalloid | Definition, Elements, & Facts. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/metalloid
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

