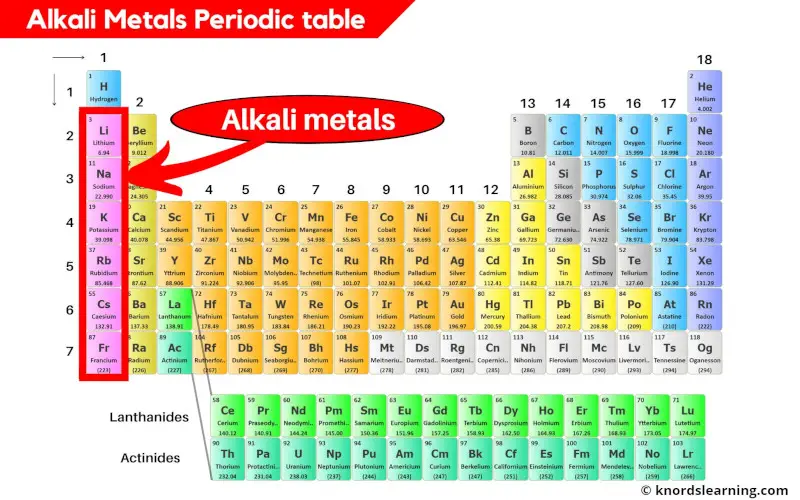
Alkali metals are highly reactive, soft and shiny metals that are located in group 1 of the periodic table.
Alkali metals includes;
- Lithium (Li)
- Sodium (Na)
- Potassium (K)
- Rubidium (Rb)
- Cesium (Cs) and
- Francium (Fr)
Well there are more interesting things to know about the alkali metals of periodic table.
So let’s dive right into it.
Table of contents
- What are Alkali metals? And why are they called so?
- Why are Alkali metals highly reactive?
- Most reactive Alkali metal on periodic table
- Reactivity of Alkali metals
- List of Alkali metals
- Electronic configuration of Alkali metals
What are Alkali Metals? And Why are they called so?
The alkali metals are the metals which form alkalis (i.e strong base) on reacting with water.

And as these metals (Li, Na, K, Rb, Cs, and Fr) form alkalis (i.e. strong base), they are known as alkali metals.
Let’s take a few examples to understand this chemical reaction.
Lithium:
Lithium (Li) reacts with water (H2O) and forms lithium hydroxide (LiOH) which is a strong base (or alkaline solution).
Sodium:
Sodium (Na) reacts with water (H2O) and forms sodium hydroxide (NaOH) which is also a strong base (or alkaline solution).
And similarly all the other group 1 elements form the alkaline solution when they react with water.
Hence they are called alkali metals.
Why are Alkali metals highly reactive?
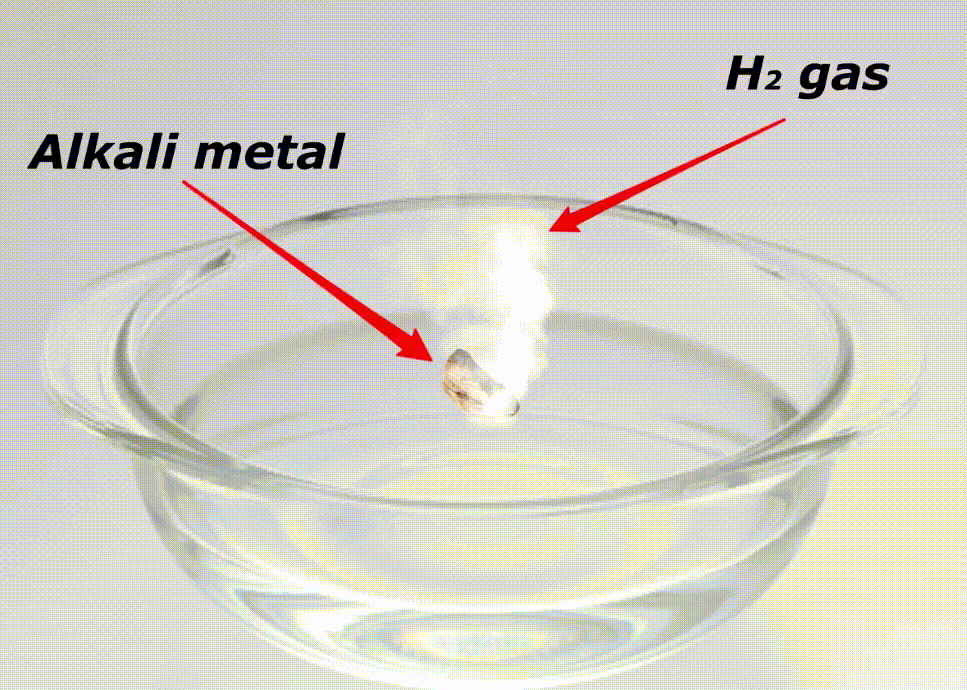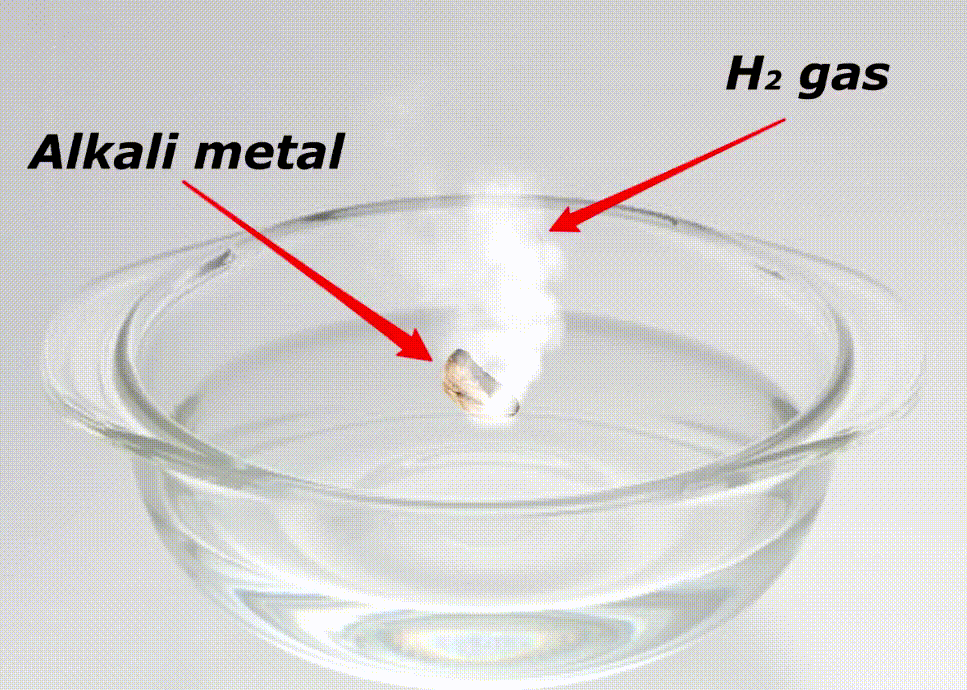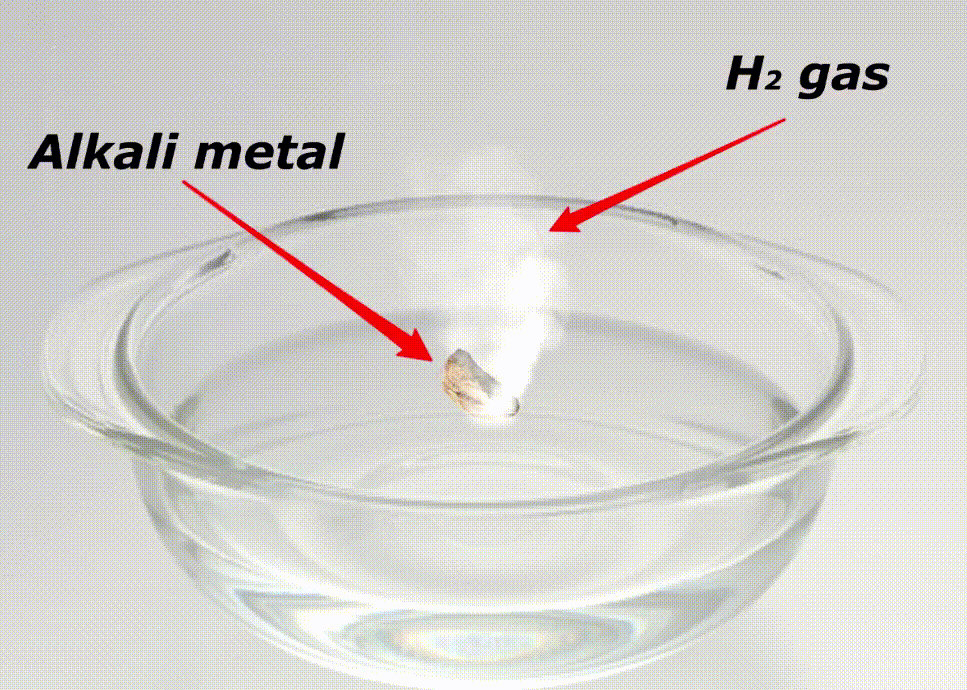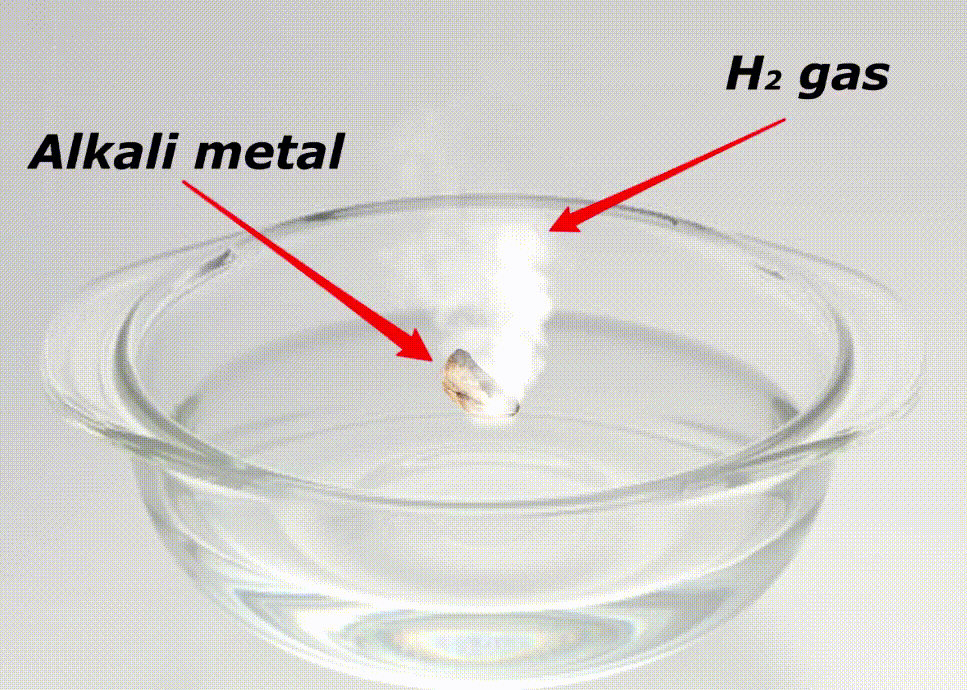
Alkali metals are highly reactive because of many reasons like;
- Only one valence electron
- Large atomic radius
- Very less ionization energy
Let me explain these reasons in short, so that you can get the exact idea behind the high reactivity of alkali metals.
Only one valence electron: As alkali metals have only one valence electron, they can easily lose this electron during a chemical reaction.
And as they lose this electron very easily, they are highly reactive.
Large atomic radius: Also the alkali metals have large atomic radius as compared to other elements on the right side of alkali metals.
Because of the larger atomic radius, the force of attraction between the nucleus and valence electron is less.
And because of this less force of attraction, the outermost electron can be easily lost during the chemical reaction.
Hence, alkali metals are also more reactive because of their larger atomic radius.
Very less ionization energy: First of all, ionization energy is the minimum energy required to remove the electron from the gaseous state or ion.
Now, alkali metals have large atomic radius as well as they have only one valence electron. Hence, very less energy is required to remove the electron from its orbit.
Thus alkali metals have very less ionization energy. And because of this, the alkali metals show higher reactivity.
Here is a video showing the reactivity of alkali metals with water.
Most reactive Alkali metal on Periodic table
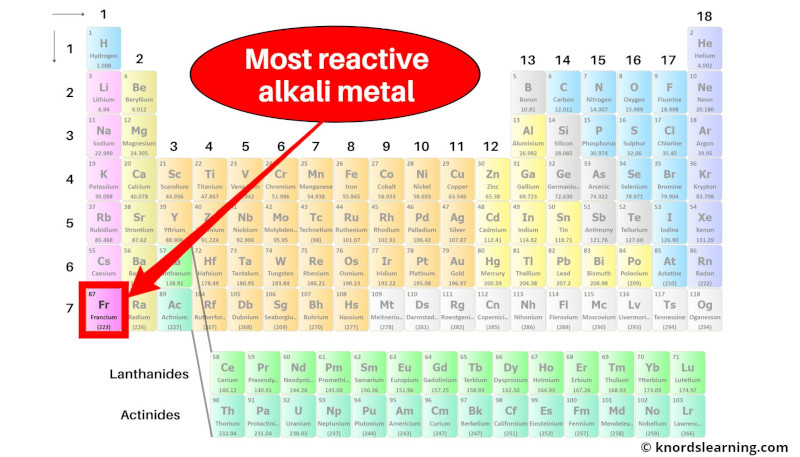
Element 87, Francium (Fr) is considered as the most reactive alkali metal on the entire periodic table.
But what is the reason behind this?
Francium (Fr) is the alkali metal that has the largest atomic radius.
Also there is only 1 valence electron in francium.
So because of this, the loss of electrons becomes very easy during a chemical reaction.
Hence, francium is the most reactive alkali metal.
Reactivity of Alkali metals
The reactivity of alkali metals increases as we move down the group in periodic table.
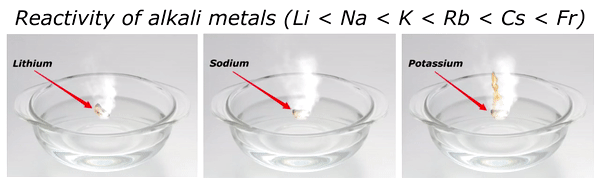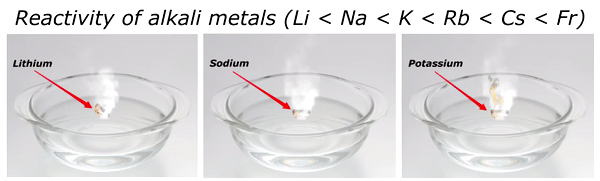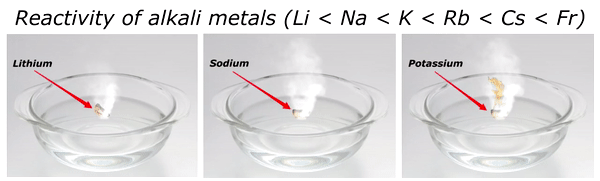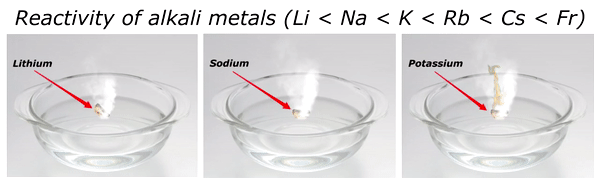
The reactivity series of alkali metals is shown in the above image.
You can see in the above image that lithium (Li) is the least reactive alkali metal and as we move further downward in the group, the reactivity of alkali metals increases. And in this way francium (Fr) is the most reactive alkali metal.
List of Alkali metals
The complete list of alkali metals is mentioned below.
| Atomic number | Name and symbol of element |
|---|---|
| 3 | Lithium (Li) |
| 11 | Sodium (Na) |
| 19 | Potassium (K) |
| 37 | Rubidium (Rb) |
| 55 | Cesium (Cs) |
| 87 | Francium (Fr) |
Electronic configuration of Alkali metals
The electronic configuration of alkali metals is listed below.
| Element | Electronic configuration |
|---|---|
| Lithium | [He] 2s1 |
| Sodium | [Ne] 3s1 |
| Potassium | [Ar] 4s1 |
| Rubidium | [Kr] 5s1 |
| Cesium | [Xe] 6s1 |
| Francium | [Rn] 7s1 |
External resources:
- Alkali metals. (n.d.). Alkali Metals – OpenLearn – Open University. https://science-maths-technology/science/chemistry/alkali-metals
- Group 1: Hydrogen and the Alkali Metals. (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_s-Block_Elements/Group__1%3A_The_Alkali_Metals
- of Response and Restoration, US GOV, N. O. (n.d.). Metals, Alkali, Very Active | CAMEO Chemicals | NOAA. Metals, Alkali, Very Active | CAMEO Chemicals | NOAA. https://cameochemicals.noaa.gov/react/21
- Dye, J. L. (2015, March 13). The alkali metals: 200 years of surprises. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 373(2037), 20140174. https://doi.org/10.1098/rsta.2014.0174
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/periodic_main1.htm
- Information on Alkali Metals – Stanford Environmental Health & Safety. (n.d.). Information on Alkali Metals – Stanford Environmental Health & Safety. http://ehs.stanford.edu/reference/information-alkali-metals
- Alkali Metal Reactivity | Chemdemos. (n.d.). Alkali Metal Reactivity | Chemdemos. http://chemdemos.uoregon.edu/demos/Alkali-Metal-Reactivity
- Alkali metal – Wikipedia. (2012, March 24). Alkali Metal – Wikipedia. https://en.wikipedia.org/wiki/Alkali_metal
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.