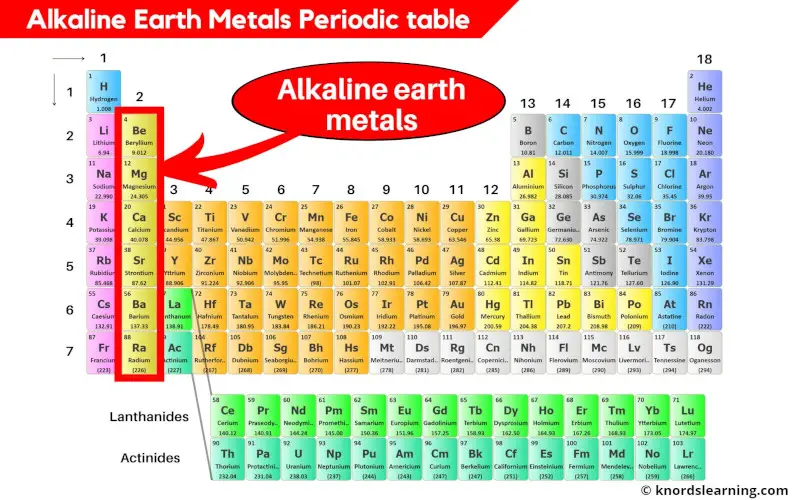
Alkaline earth metals are the elements located in group 2 of the periodic table. These elements are;
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba) and
- Radium (Ra)
Let’s see a few important and interesting things about alkaline earth metals of the periodic table.
Table of contents
- What are alkaline earth metals?
- Alkaline earth metals list
- Common things about alkaline earth metals
- Electronic configuration of alkaline earth metals
What are alkaline earth metals?
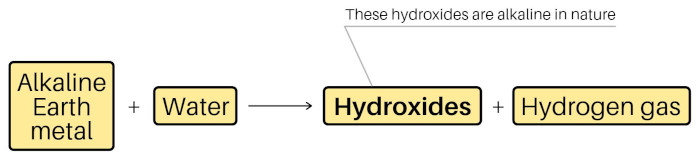
Alkaline earth metals (Be, Mg, Ca, Sr, Ba and Ra) are the metals that form alkaline hydroxides {Ba(OH)2 , Mg(OH)2 , Ca(OH)2 , etc} on reacting with water, and the oxide minerals of these metals (BeO, beryl, MgO, magnesite, etc) are found from the earth’s crust.
For example:
Beryllium, Magnesium, Calcium, Strontium, Barium and Radium, when react with water, they form hydroxides. And these hydroxides are alkaline in nature (or basic in nature, that have pH > 7).
And also, the oxides of these metals (BeO, beryl, MgO, magnesite, etc) are obtained from the earth’s crust.
Hence, these metals of group 2 are called alkaline earth metals.
Alkaline Earth Metals list
List of alkaline earth metals is mentioned below.
| Atomic number | Name and symbol of element |
|---|---|
| 4 | Beryllium (Be) |
| 12 | Magnesium (Mg) |
| 20 | Calcium (Ca) |
| 38 | Strontium (Sr) |
| 56 | Barium (Ba) |
| 88 | Radium (Ra) |
Common things about Alkaline Earth Metals
Alkaline earth metals have common physical properties as well as chemical properties.
- The alkaline earth metals have a similar appearance. They are silvery-white and shiny in appearance.

You can see the shiny silvery-white appearance of alkaline earth metals in the above image.
- The alkaline earth metals have similar chemical properties because they all have two valence electrons.
As the alkaline earth metals have 2 valence electrons and they lose these 2 valence electrons during a chemical reaction.
| Alkaline earth metals | Atomic number (Z) | Electrons arrangement |
|---|---|---|
| Beryllium (Be) | 4 | 2, 2 |
| Magnesium (Mg) | 12 | 2, 8, 2 |
| Calcium (Ca) | 20 | 2, 8, 8, 2 |
| Strontium (Sr) | 38 | 2, 8, 18, 8, 2 |
| Barium (Ba) | 56 | 2, 8, 18, 18, 8, 2 |
| Radium (Ra) | 88 | 2, 8, 18, 32, 18, 8, 2 |
As they lose 2 electrons, they form a cation with +2 charge.
Electronic configuration of Alkaline Earth Metals
The electron configuration of alkaline earth metals is mentioned below.
| Element | Electronic configuration |
|---|---|
| Beryllium (Be) | [He] 2s2 |
| Magnesium (Mg) | [Ne] 3s2 |
| Calcium (Ca) | [Ar] 4s2 |
| Strontium (Sr) | [Kr] 5s2 |
| Barium (Ba) | [Xe] 6s2 |
| Radium (Ra) | [Rn] 7s2 |
External resources:
- Alkaline earths (n.d.). http://hyperphysics.phy-astr.gsu.edu/hbase/pertab/alkear.html
- US GOV, N. O. (n.d.). ALKALINE EARTH METAL DISPERSION | CAMEO Chemicals | NOAA. ALKALINE EARTH METAL DISPERSION | CAMEO Chemicals | NOAA. https://cameochemicals.noaa.gov/chemical/19037
- The Alkaline Earth Metals. (n.d.). The Alkaline Earth Metals. https://bluebox.creighton.edu/demo/modules/en-boundless-old/www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/metals-20/metals-145/the-alkaline-earth-metals-565-6854/index.html
- Foundation, C. (n.d.). CK12-Foundation. CK12-Foundation. https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/6.10/primary/lesson/alkaline-earth-metals-chem/
- 6.10: Alkaline Earth Metals. (2016, June 27). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/06%3A_The_Periodic_Table/6.10%3A_Alkaline_Earth_Metals
- Alkaline-earth metal | Properties, List, & Reactivity. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/alkaline-earth-metal
- Alkaline earth metal – Wikipedia. (2012, November 1). Alkaline Earth Metal – Wikipedia. https://en.wikipedia.org/wiki/Alkaline_earth_metal
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. http://www.angelo.edu/faculty/kboudrea/periodic/periodic_main2.htm
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

