
Molar mass of CaF2 (Calcium fluoride) is 78.074 g/mol.
Well, now you have come to know the molar mass of CaF2.
But how can you get this value?
Let me show you the calculation to get the molar mass of CaF2 (Calcium fluoride).
You can also refer to this one minute video which will show you the simple steps to calculate the molar mass of any compounds.
CaF2 (Calcium fluoride) Molar Mass Calculation
If you have a periodic table with you, then you can easily calculate the molar mass of CaF2 (Calcium fluoride).
Because the molar mass of any molecule (or compound) can be calculated by simply adding the molar masses of individual atoms.
Now here we have to find the molar mass of CaF2 (Calcium fluoride).
So for that, have a look at the periodic table given below.
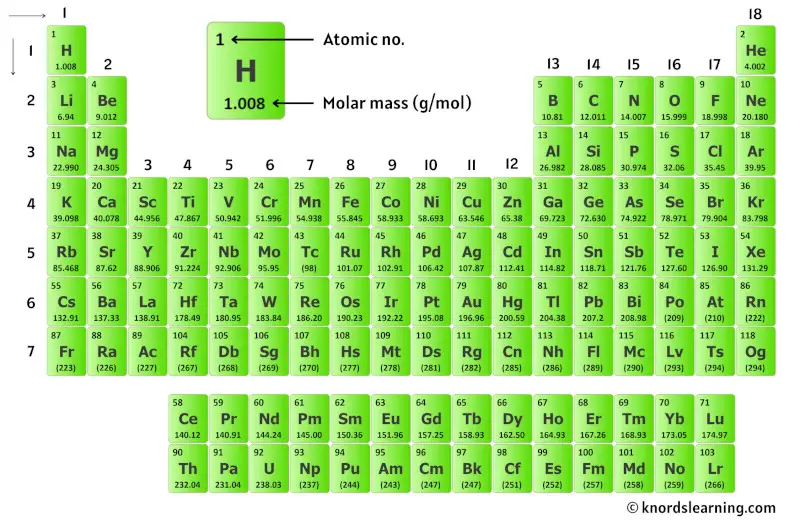
You can see the molar mass value of all the atoms from this periodic table.
Now in CaF2, there is 1 Calcium atom and 2 Fluorine atoms.
So let’s look at the molar mass of Calcium and Fluorine from the above periodic table.
You can see that;
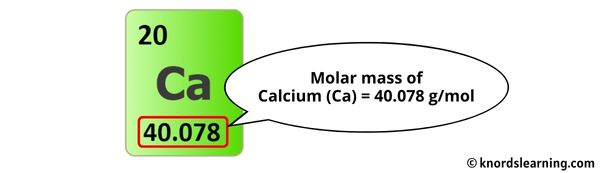
The molar mass of Calcium is 40.078 g/mol. [1]
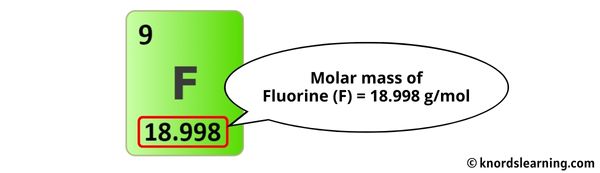
The molar mass of Fluorine is 18.998 g/mol. [2]
Now, to calculate the molar mass of CaF2, you just have to add the molar mass of all the individual atoms that are present in CaF2.
You can see that in CaF2, there is 1 Calcium atom and 2 Fluorine atoms.
So, Molar mass of CaF2 = Molar mass of 1 Calcium (Ca) atom + Molar mass of 2 Fluorine (F) atoms.
= 40.078 + (18.998) 2
= 40.078 + 37.996
= 78.074 g/mol
Hence the Molar mass of CaF2 is 78.074 g/mol.
I hope you have understood the short and simple calculation for finding the molar mass of CaF2.
Remember
- In some books, you may see the unit of molar mass as grams/mole or g/mole. But all these units (i.e g/mol, grams/mole and g/mole) are the same.
- Always follow the calculation order to avoid any mistakes in calculation. First solve the brackets, then multiplications and at last do the final addition.
- And don’t forget to put the unit g/mol to your final calculated molar mass.
Check out other related topics for more practice;
Hydrogen iodide (HI) Molar Mass
C2H2 (Ethyne) Molar Mass
Alum Molar Mass
Acetaminophen Molar Mass
Glycine (C2H5NO2) Molar Mass
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

