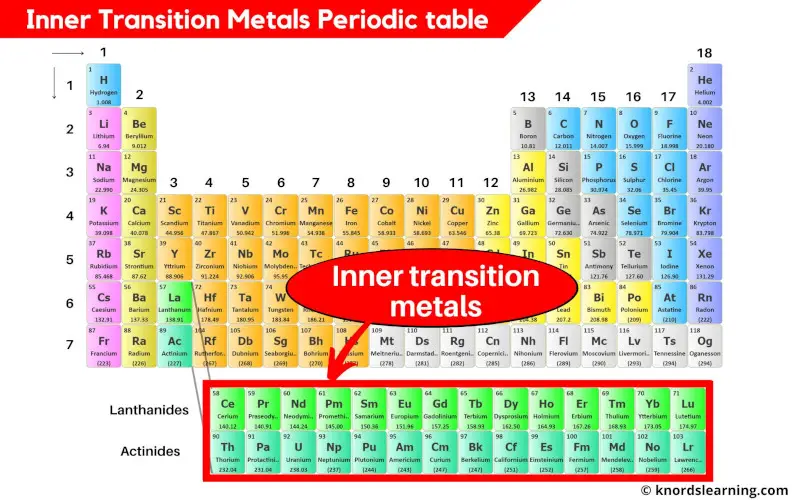
The inner transition metals are the elements that are found in the two rows which are at the bottom of the periodic table.
The inner transition metals include;
- Lanthanides (from element 58 to 71)
- Actinides (from element 90 to 103)
There are many important things to know about the inner transition elements of the periodic table.
So let’s dive right into it.
Table of contents
- Why are inner transition elements called so?
- Why are inner transition metals placed at the bottom of the periodic table?
- List of inner transition elements
- Facts about inner transition metals
Why are Inner transition elements called so?
Simply look at the name itself, you will easily get the idea.
The name “Inner transition elements” has two terms.
- “Inner” and
- “Transition elements”
So from this you can get the idea that inner transition elements have the similar properties as that of transition elements only, but they are placed in the inner section as the extension of group 3.
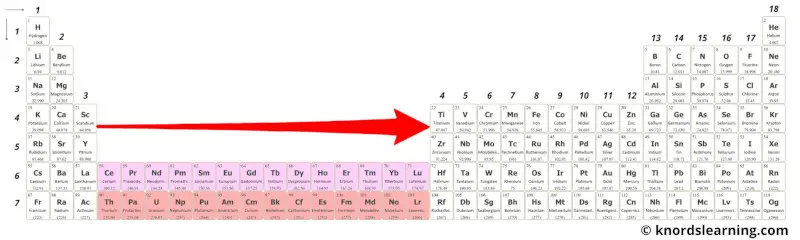
From the above image, you can see that these elements are very near to transition metals and so they have similar properties.
In other words, these elements are just the extension of group 3 on the periodic table. And because of this, they show similar properties.
Also,
These elements are placed on the periodic table as an inner part of the group 3.
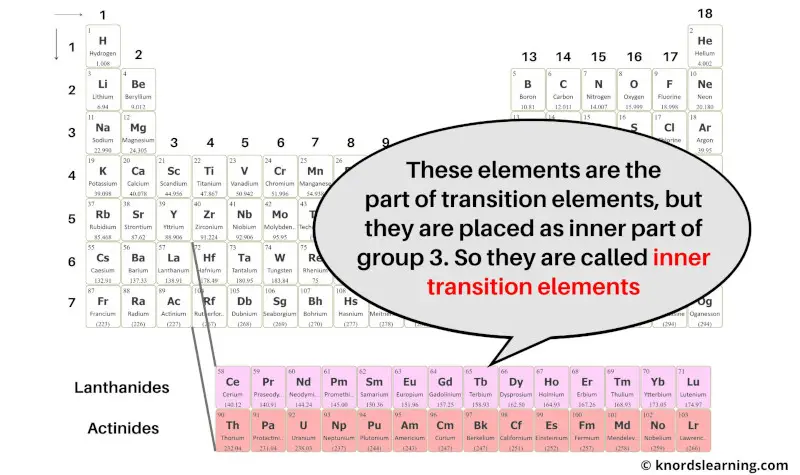
Hence because of these two reasons, these elements are known as inner transition elements.
Why are inner transition metals placed at the bottom of the periodic table?
There are 2 reasons behind this;
- To make the periodic table look short
- These elements have valence electrons in f-orbital
Let me explain this in short.
Just imagine a periodic table that has inner transition metals placed just besides the transition metals.
Then the periodic table would look something like this.
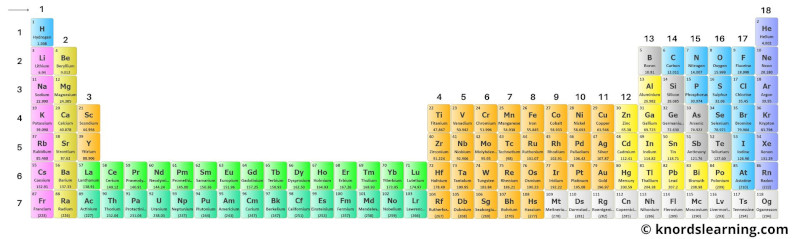
This periodic table looks very long. Isn’t it?
So in order to make the periodic table short and to make it perfectly fit on A4 size paper, the two rows (i.e lanthanides and actinides) are placed separately at the bottom of the periodic table.
Now, these elements of the bottom rows (lanthanides and actinides) have similar chemical properties as they have their valence electrons in f-orbital.
As these elements have similar chemical properties, it makes sense to place them in a separate block at the bottom of the periodic table.
List of Inner transition metals
List of inner transition metals is mentioned below.
| Atomic number | Name and symbol of element |
|---|---|
| 58 | Cerium (Ce) |
| 59 | Praseodymium (Pr) |
| 60 | Neodymium (Nd) |
| 61 | Promethium (Pm) |
| 62 | Samarium (Sm) |
| 63 | Europium (Eu) |
| 64 | Gadolinium (Gd) |
| 65 | Terbium (Tb) |
| 66 | Dysprosium (Dy) |
| 67 | Holmium (Ho) |
| 68 | Erbium (Er) |
| 69 | Thulium (Tm) |
| 70 | Ytterbium (Yb) |
| 71 | Lutetium (Lu) |
| 90 | Thorium (Th) |
| 91 | Protactinium (Pa) |
| 92 | Uranium (U) |
| 93 | Neptunium (Np) |
| 94 | Plutonium (Pu) |
| 95 | Americium (Am) |
| 96 | Curium (Cm) |
| 97 | Berkelium (Bk) |
| 98 | Californium (Cf) |
| 99 | Einsteinium (Es) |
| 100 | Fermium (Fm) |
| 101 | Mendelevium (Md) |
| 102 | Nobelium (No) |
| 103 | Lawrencium (Lr) |
Facts about Inner transition elements
Interesting facts about inner transition metals are mentioned below.
- The inner transition elements are radioactive in nature and most of them are artificially prepared in the laboratory.
- The valence electron of all the inner transition elements lies in the f-orbital.
- Few lanthanides are so soft that they can be cut with a kitchen knife.
- Lanthanide elements are found naturally from the earth’s crust.
External resources:
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. http://www.angelo.edu/faculty/kboudrea/periodic/trans_inner_transition.htm
- CHEM765 – F-Block Elements | Department of Chemistry. (n.d.). CHEM765 – F-Block Elements | Department of Chemistry. https://www.chem.upenn.edu/node/9548
- F-block element chemistry. (n.d.). F-block Element Chemistry | Sustainable Synthesis and Catalysis | University of Leicester. https://le.ac.uk/chemistry/research/sustainable-synthesis-and-catalysis/f-block-element-chemistry
- f-Block Elements. (2014, May 1). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/4_f-Block_Elements
- F-block elements. (2022, September 7). Nature. https://www.nature.com/collections/highbeiiaj
- General Chemistry/Chemistries of Various Elements/Inner Transition Metals – Wikibooks, open books for an open world. (n.d.). General Chemistry/Chemistries of Various Elements/Inner Transition Metals – Wikibooks, Open Books for an Open World. https://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Inner_Transition_Metals
- Transition metal | Definition, Properties, Elements, & Facts. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/transition-metal
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

