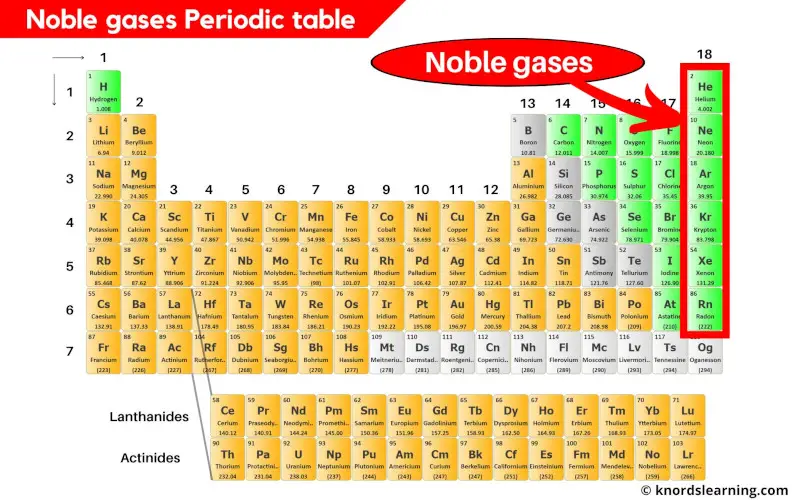
Noble gases (also known as inert gases) are the elements on the periodic table that are located in the last column (group 18) on the periodic table.
There are 6 known noble gases on the periodic table.
- Helium (He)
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe) and
- Radon (Rn)
Noble gases are highly unreactive which means they do not easily react with any other elements.
Oganesson (Og) is a laboratory made synthetic element and it is predicted to have similar properties as that of noble gases. But it shows some reactivity unlike noble gases.
Well there are a lot more things to know about noble gases.
So let’s dive right into it.
Table of contents
- What are Noble Gases? And why are they called so?
- List of noble gases
- Electronic configuration of noble gases
- Facts about noble gases
What are Noble Gases? And why are they called so?
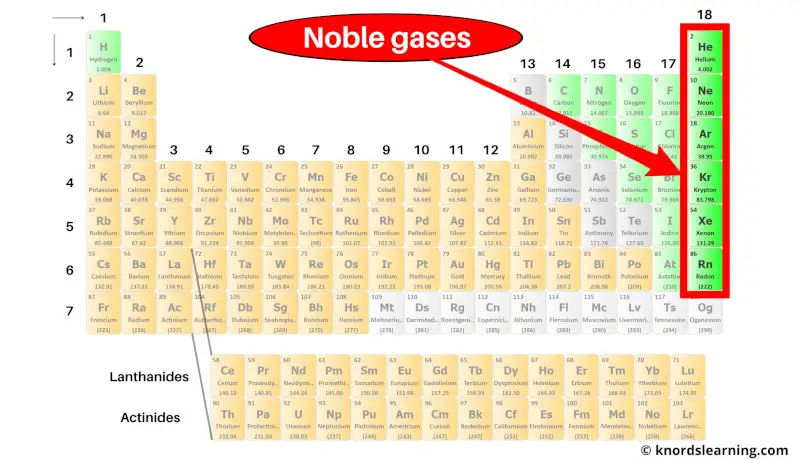
The 6 chemical elements present in the group 18 of the periodic table have completely filled outer orbits.
For example;
Helium has 2 electrons in its outer orbit (that means it has a stable duplet).
Similarly; neon, argon, krypton, xenon and radon have 8 electrons in their outermost orbits (that means they have a stable octet).
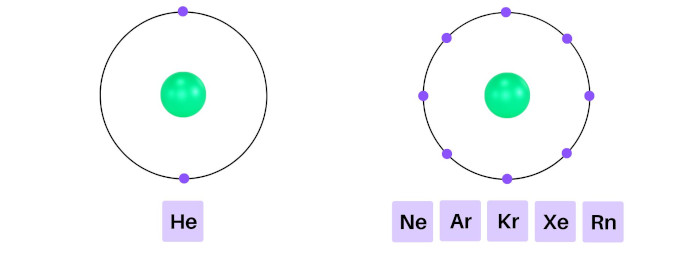
As these elements have completely filled outer shells, they are highly unreactive to other elements.
And as they are unreactive to other elements, they are known as noble gases (or inert gases).
In the past, chemists thought that the noble gases were so stable that they could not react with any other elements. But later on they found that few noble gases form compounds with other elements.
Noble gases can form compounds with highly reactive nonmetal fluorine;
Such compounds are XeF4, XeF6, RnF2, etc.
List of Noble Gases
List of noble gases of the periodic table is mentioned below.
| Atomic number | Name and symbol of element |
| 2 | Helium (He) |
| 10 | Neon (Ne) |
| 18 | Argon (Ar) |
| 36 | Krypton (Kr) |
| 54 | Xenon (Xe) |
| 86 | Radon (Rn) |
Electronic configuration of Noble Gases
Helium has an electronic configuration of 1s2, while rest of the other noble gasses have the general outer electronic configuration of ns2 np6, where n is the number of outer orbits.
The electron configuration and electron shell arrangement of the noble gases are mentioned below.
| Noble gases | Atomic number (Z) | Electronic configuration | Electrons arrangement |
|---|---|---|---|
| Helium (He) | 2 | 1s1 | 2, 2 |
| Neon (Ne) | 10 | [He] 2s2 2p6 | 2, 8 |
| Argon (Ar) | 18 | [Ne] 3s2 3p6 | 2, 8, 8 |
| Krypton (Kr) | 36 | [Ar] 3d10 4s2 4p6 | 2, 8, 18, 8 |
| Xenon (Xe) | 54 | [Kr] 4d10 5s2 5p6 | 2, 8, 18, 18, 8 |
| Radon (Rn) | 86 | [Xe] 4f14 5d10 6s2 6p6 | 2, 8, 18, 32, 18, 8 |
Facts about Noble Gases
Facts about noble gases are listed below.
- Helium is present in large quantities in stars and helium is the 2nd most abundant element found in the entire universe (around 24%)
- Helium can be obtained from decay of radioactive elements like uranium and thorium.
- Out of all the known noble gases, argon was discovered first.
- Xenon is present in very less quantity in earth’s atmosphere. It’s only about 0.087 ppm (parts per million).
- Xenon is also found in large quantities on the planet Jupiter.
External resources:
- Noble gas – Wikipedia. (2014, May 30). Noble Gas – Wikipedia. https://en.wikipedia.org/wiki/Noble_gas
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – THE NOBLE GASES. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – THE NOBLE GASES. https://pubsapp.acs.org/cen/80th/noblegases.html?
- Noble Gases. (n.d.). Noble Gases. http://butane.chem.uiuc.edu/pshapley/GenChem1/L6/1.html
- Noble Gases. (n.d.). NASA/ADS. https://doi.org/10.1016/B0-08-043751-6/01066-5
- Noble gas | Definition, Elements, Properties, Characteristics, & Facts. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/noble-gas
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.