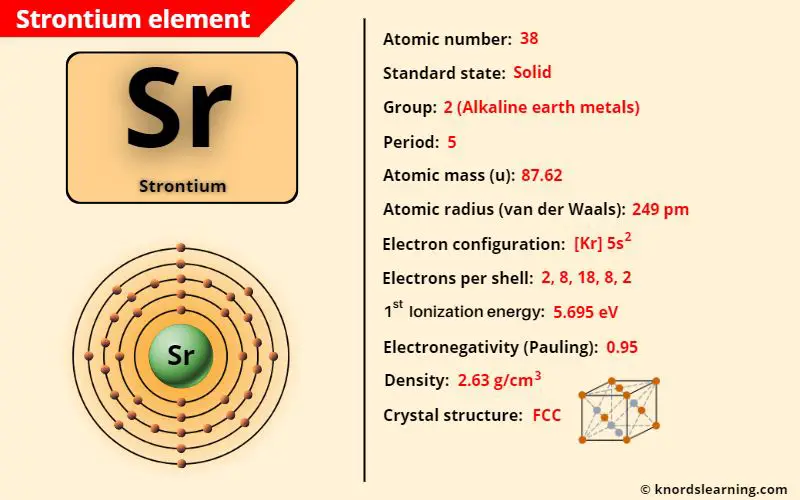
Strontium element (Sr) is in group 2 and period 5 of a periodic table. Strontium is in the s-block and it is classified as an alkaline earth metal on the periodic table.
There is a lot more information related to strontium which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Strontium element (Information Table)
- Strontium element in Periodic table
- Facts about Strontium
- Properties of Strontium
- Uses of Strontium
Strontium Element (Information Table)
The important data related to strontium element is given in the table below.
| Appearance of strontium | Silvery surface with yellow tint |
| Atomic number of strontium | 38 |
| Symbol of strontium | Sr |
| Atomic mass of strontium | 87.62 u |
| Protons, Neutrons & Electrons in strontium | Protons: 38, Neutrons: 50, Electrons: 38 |
| State of strontium (at STP) | Solid |
| Group number of strontium in periodic table | 2 |
| Period number of strontium in periodic table | 5 |
| Block of strontium in periodic table | s-block |
| Category of strontium | Alkaline earth metals |
| Bohr model or Electrons per shell or Electrons arrangement in strontium | 2, 8, 18, 8, 2 |
| Electron configuration of strontium | [Kr] 5s2 |
| Orbital diagram of strontium |  |
| Valence electrons in strontium | 2 |
| Electronegativity of strontium (on pauling scale) | 0.95 |
| Atomic radius of strontium (van der Waals radius) | 249 picometers |
| Density of strontium | 2.63 g/cm3 |
| 1st ionization energy of strontium | 5.695 eV |
| Main isotope of strontium | 88Sr |
| Melting point of strontium | 1050 K or 777 °C or 1431 °F |
| Boiling point of strontium | 1650 K or 1377 °C or 2511 °F |
| Crystal structure of strontium | Face Centered Cubic (FCC) |
| Discovery of strontium | By William Cruickshank in 1787 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Strontium element in Periodic table
The Strontium element (Sr) has the atomic number 38 and is located in group 2 and period 5. Strontium is in solid state at STP and it is classified as an alkaline earth metal on the periodic table.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about strontium
Here are a few interesting facts about the strontium element.
- Strontium is the 15th most abundant element present in the earth’s crust.
- The concentration of strontium in the earth’s crust is around 360 ppm.
- Around 3 lakh tons of strontium is produced annually in the entire world.
- Strontium is a reactive metal and hence it is kept in the mineral oil or kerosene.
- Strontianite and Celestite are the two ores from which most of the strontium is obtained.
- The leading producers of strontium are China and Spain.
Properties of strontium
Here is a list of some physical properties and chemical properties of strontium.
Physical properties of strontium
- Strontium is a soft metal and it has a silvery surface with a yellowish tint.
- Strontium has an atomic mass 87.62 u and its density is 2.63 g/cm3.
- Strontium has stable isotopes as well as radioactive isotopes, and out of these isotopes, the most abundant isotope is 88Sr (which has an abundance of 82.5%).
- The melting point and boiling point of strontium is 1050 K and 1650 K respectively.
Chemical properties of strontium
- Strontium is reactive metal and hence it is always found as a compound with other elements in the earth’s crust.
- Strontium burns in the air with a bright red flame.
- Strontium reacts with water to form strontium hydroxide and liberates hydrogen gas.
Uses of strontium
Here are some uses of the strontium element.
- Strontium is used in flares as well as fireworks because it burns with a bright red flame.
- Strontium titanate is used in gemstones as it has a high refractive index.
- Strontium chloride hexahydrate is used in toothpaste for sensitive teeth.
- The radioactive isotope of strontium (89Sr) is used in treating bone cancer.
- Strontium aluminate is used in toys that glow in dark (because it has a property to glow in dark).
External resources:
- Strontium – Wikipedia. (2022, December 7). Strontium – Wikipedia. https://en.wikipedia.org/wiki/Strontium
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/38.shtml
- Atomic Data for Strontium (Sr). (n.d.). Atomic Data for Strontium (Sr). https://physics.nist.gov/PhysRefData/Handbook/Tables/strontiumtable1.htm
- Sansonetti, J. E., & Martin, W. C. (2005, December). Handbook of Basic Atomic Spectroscopic Data. Journal of Physical and Chemical Reference Data, 34(4), 1559–2259. https://doi.org/10.1063/1.1800011
- Bondi, A. (1964, March). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), 441–451. https://doi.org/10.1021/j100785a001
- Holden, et al. (2018, December 1). IUPAC Periodic Table of the Elements and Isotopes (IPTEI) for the Education Community (IUPAC Technical Report). Pure and Applied Chemistry, 90(12), 1833–2092. https://doi.org/10.1515/pac-2015-0703
- Zhang, et al. (2011, January 11). Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. Journal of Chemical & Engineering Data, 56(2), 328–337. https://doi.org/10.1021/je1011086
- Strontium Statistics and Information | U.S. Geological Survey. (n.d.). Strontium Statistics and Information | U.S. Geological Survey. https://www.usgs.gov/centers/national-minerals-information-center/strontium-statistics-and-information
- Strontium – Element information, properties and uses | Periodic Table. (n.d.). Strontium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/38/strontium
- Prohaska, T., et al. (2022, May 1). Standard atomic weights of the elements 2021 (IUPAC Technical Report). Pure and Applied Chemistry, 94(5), 573–600. https://doi.org/10.1515/pac-2019-0603
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Kaye, G W.C., & Laby, T H. Tables of physical and chemical constants. 15th Edition. United States.
- It’s Elemental – The Element Strontium. (n.d.). It’s Elemental – the Element Strontium. https://education.jlab.org/itselemental/ele038.html
- P. (n.d.). Strontium | Sr (Element) – PubChem. Strontium | Sr (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Strontium
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

