
Gallium element (Ga) is in group 13 and period 4 of a periodic table. Gallium is in the p-block and it is classified as a post-transition element on the periodic table.
There is a lot more information related to gallium which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Gallium element (Information Table)
- Gallium element in Periodic table
- Facts about Gallium
- Properties of Gallium
- Uses of Gallium
Gallium Element (Information Table)
The important data related to gallium element is given in the table below.
| Appearance of gallium | Silvery blue in color |
| Atomic number of gallium | 31 |
| Symbol of gallium | Ga |
| Atomic mass of gallium | 69.723 u |
| Protons, Neutrons & Electrons in gallium | Protons: 31, Neutrons: 39, Electrons: 31 |
| State of gallium (at STP) | Solid |
| Group number of gallium in periodic table | 13 |
| Period number of gallium in periodic table | 4 |
| Block of gallium in periodic table | p-block |
| Category of gallium | Post transition element (boron group) |
| Bohr model or Electrons per shell or Electrons arrangement in gallium | 2, 8, 18, 3 |
| Electron configuration of gallium | [Ar] 3d10 4s2 4p1 |
| Orbital diagram of gallium | 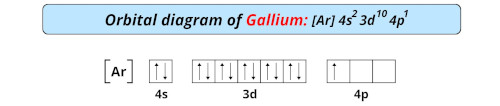 |
| Valence electrons in gallium | 3 |
| Electronegativity of gallium (on pauling scale) | 1.81 |
| Atomic radius of gallium (van der Waals radius) | 187 picometers |
| Density of gallium | 5.904 g/cm3 |
| 1st ionization energy of gallium | 5.999 eV |
| Main isotope of gallium | 69Ga |
| Melting point of gallium | 302.9 K or 29.7 °C or 85.5 °F |
| Boiling point of gallium | 2673 K or 2400 °C or 4352 °F |
| Crystal structure of gallium | Orthorhombic |
| Discovery of gallium | By Lecoq de Boisbaudran in 1875 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Gallium element in Periodic table
The Gallium element (Ga) has the atomic number 31 and is located in group 13 and period 4. Gallium is a metal and it is classified as a post-transition element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about gallium
Here are a few interesting facts about gallium element.
- Gallium was given its name from the Latin word “Gallia”.
- Gallium is a soft metal and it can be cut with a knife.
- Gallium is the 34th most abundant element found from the earth’s crust.
- The quantity of gallium in the earth’s crust is around 0.0019% by weight.
- Gallium shows an expansion on freezing. Hence gallium should not be stored in glass containers.
- There is a large difference between the melting point and boiling point of gallium. Its melting point is 29.7 °C, which is close to room temperature, while its boiling point is 2400 °C.
- Gallium melts in your hand due to the heat of the body.
Properties of gallium
Here is a list of some physical properties and chemical properties of gallium.
Physical properties of gallium
- Gallium is a soft metal and it has a silvery blue appearance.
- Gallium becomes brittle at lower temperatures.
- Gallium shows expansion in its volume on freezing.
- The melting point of gallium is 29.7 °C and its booking point is 2400 °C.
- There are many isotopes of gallium, but out of those isotopes, the most abundant isotope is 69Ga (it has an abundance of around 60%).
Chemical properties of gallium
- Gallium is a less reactive element but it slowly forms an oxide layer on it when kept open in the air.
- Gallium reacts with group 16 elements (chalcogens) at higher temperatures.
- The electron configuration of gallium is [Ar] 3d10 4s2 4p1 and it is classified as a post-transition element on the periodic table.
- Gallium also shows a reaction with oxygen at higher temperature and this forms a gallium oxide.
- The thin oxide layer formed on the pure gallium protects it from attack of mineral acids.
Uses of gallium
Here are some uses of the gallium element.
- Gallium arsenide is a compound of gallium that is used in microwave as well as infrared circuits.
- Gallium arsenide produces a laser light when the electric current is passed through it.
- Gallium is also used in equipment like barometers, high temperature thermometers, etc.
- Gallium nitride is used in pressure sensors as well as blu-ray technology.
- Gallium is also used to make alloys that require a low melting temperature.
External resources:
- Gallium – Element information, properties and uses | Periodic Table. (n.d.). Gallium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/31/gallium
- Gallium – Wikipedia. (2009, July 7). Gallium – Wikipedia. https://en.wikipedia.org/wiki/Gallium
- It’s Elemental – The Element Gallium. (n.d.). It’s Elemental – the Element Gallium. https://education.jlab.org/itselemental/ele031.html
- P. (n.d.). Gallium | Ga (Element) – PubChem. Gallium | Ga (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Gallium
- Gallium Statistics and Information | U.S. Geological Survey. (n.d.). Gallium Statistics and Information | U.S. Geological Survey. https://www.usgs.gov/centers/national-minerals-information-center/gallium-statistics-and-information
- Gallium | Ga | ChemSpider. (n.d.). Gallium | Ga | ChemSpider. http://www.chemspider.com/Chemical-Structure.4514603.html?rid=57779e6a-2093-4c32-aebf-00757828b8a4&page_num=0
- Atomic Data for Gallium (Ga). (n.d.). Atomic Data for Gallium (Ga). https://physics.nist.gov/PhysRefData/Handbook/Tables/galliumtable1.htm
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/31.shtml
- Possolo, et al. (2018, January 4). Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report). Pure and Applied Chemistry, 90(2), 395–424. https://doi.org/10.1515/pac-2016-0402
- Emsley, J. (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. United Kingdom: OUP Oxford.
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Electronic structure of the elements. (2000, March). The European Physical Journal C, 15(1–4), 78–79. https://doi.org/10.1007/bf02683401
- James A. M. & Lord M. P. (1992). Macmillan’s chemical and physical data. Macmillan.
- Bedford, et al. (1996, April 1). Recommended values of temperature on the International Temperature Scale of 1990 for a selected set of secondary reference points. Metrologia, 33(2), 133–154. https://doi.org/10.1088/0026-1394/33/2/3
- Allred, A. (1961, June). Electronegativity values from thermochemical data. Journal of Inorganic and Nuclear Chemistry, 17(3–4), 215–221. https://doi.org/10.1016/0022-1902(61)80142-5
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.
