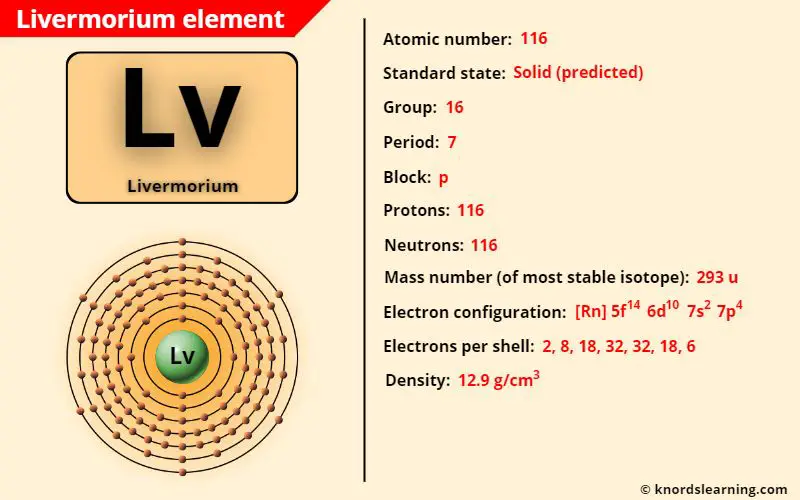
Livermorium element (Lv) is in group 16 and period 7 of a periodic table. Livermorium is in the p-block and it is classified as a radioactive synthetic element on the periodic table.
There is a lot more information related to livermorium which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Livermorium element (Information Table)
- Livermorium element in Periodic table
- Facts about Livermorium
- Properties of Livermorium
- Uses of Livermorium
Livermorium Element (Information Table)
The important data related to livermorium element is given in the table below.
| Atomic number of livermorium | 116 |
| Symbol of livermorium | Lv |
| Atomic mass of livermorium (most stable isotope) | 293 u |
| Protons in livermorium | 116 |
| Electrons in livermorium | 116 |
| State of livermorium (at STP) | Solid (predicted) |
| Group number of livermorium in periodic table | 16 |
| Period number of livermorium in periodic table | 7 |
| Block of livermorium in periodic table | p-block |
| Category of livermorium | Synthetic element |
| Bohr model or Electrons per shell or Electrons arrangement in livermorium | 2, 8, 18, 32, 32, 18, 6 |
| Electron configuration of livermorium | [Rn] 5f14 6d10 7s2 7p4 |
| Orbital diagram of livermorium | 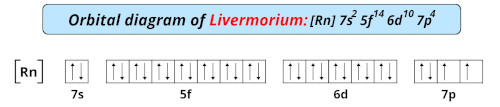 |
| Density of livermorium (predicted) | 12.9 g/cm3 |
| Main isotope of livermorium | 293Lv |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Livermorium element in Periodic table
The Livermorium element (Lv) has the atomic number 116 and is located in group 16 and period 7.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about livermorium
Here are a few interesting facts about the livermorium element.
- Livermorium was given its name from the name of the laboratory “Lawrence Livermore National laboratory”, which is in California.
- Livermorium is an artificially made element and it is not available naturally.
- All the known isotopes of livermorium are radioactive in nature.
- 293Lv is the most stable isotope and it has a half life of only 57 milliseconds.
Properties of livermorium
Here is a list of some physical properties and chemical properties of livermorium.
- Livermorium is a radioactive element and has a very short half life.
- Livermorium is predicted to have a solid state at standard temperature and pressure.
- The common predicted oxidation state of livermorium is +2.
- The predicted density of livermorium is 12.9 g/cm3 and its most stable isotope has an atomic mass 293 amu.
Uses of livermorium
Livermorium has no commercial uses due to its scarcity and highly radioactive nature. It is generally used for research work.
External resources:
- Livermorium – Element information, properties and uses | Periodic Table. (n.d.). Livermorium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/116/livermorium
- Livermorium – Wikipedia. (2013, October 4). Livermorium – Wikipedia. https://en.wikipedia.org/wiki/Livermorium
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Sansonetti, J. E., & Martin, W. C. (2005, December). Handbook of Basic Atomic Spectroscopic Data. Journal of Physical and Chemical Reference Data, 34(4), 1559–2259. https://doi.org/10.1063/1.1800011
- Bondi, A. (1964, March). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), 441–451. https://doi.org/10.1021/j100785a001
- P. (n.d.). Livermorium | Lv (Element) – PubChem. Livermorium | Lv (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Livermorium
- It’s Elemental – The Element Livermorium. (n.d.). It’s Elemental – the Element Livermorium. https://education.jlab.org/itselemental/ele116.html
- Livermorium. (n.d.). Livermorium. https://pls.llnl.gov/research-and-development/nuclear-science/project-highlights/livermorium
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/116.shtml
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

