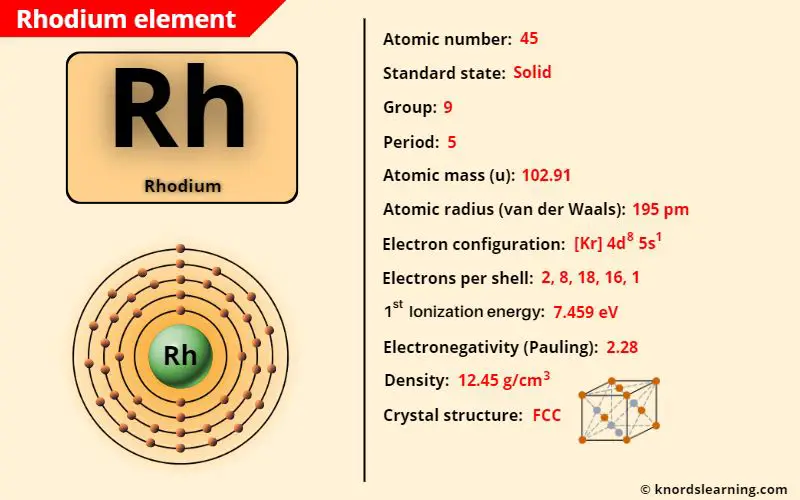
Rhodium element (Ru) is in group 9 and period 5 of a periodic table. Rhodium is in the d-block and it is classified as a transition element on the periodic table.
There is a lot more information related to rhodium which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Rhodium element (Information Table)
- Rhodium element in Periodic table
- Facts about Rhodium
- Properties of Rhodium
- Uses of Rhodium
Rhodium Element (Information Table)
The important data related to rhodium element is given in the table below.
| Appearance of rhodium | Silvery white metallic appearance |
| Atomic number of rhodium | 45 |
| Symbol of rhodium | Rh |
| Atomic mass of rhodium | 102.91 u |
| Protons, Neutrons & Electrons in rhodium | Protons: 45, Neutrons: 58, Electrons: 45 |
| State of rhodium (at STP) | Solid |
| Group number of rhodium in periodic table | 9 |
| Period number of rhodium in periodic table | 5 |
| Block of rhodium in periodic table | d-block |
| Category of rhodium | Transition metals |
| Bohr model or Electrons per shell or Electrons arrangement in rhodium | 2, 8, 18, 16, 1 |
| Electron configuration of rhodium | [Kr] 4d8 5s1 |
| Orbital diagram of rhodium | 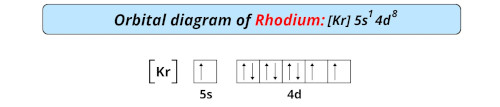 |
| Electronegativity of rhodium (on pauling scale) | 2.28 |
| Atomic radius of rhodium (van der Waals radius) | 195 picometers |
| Density of rhodium | 12.45 g/cm3 |
| 1st ionization energy of rhodium | 7.459 eV |
| Main isotope of rhodium | 103Rh |
| Melting point of rhodium | 2237 K or 1964 °C or 3567 °F |
| Boiling point of rhodium | 3968 K or 3695 °C or 6683 °F |
| Crystal structure of rhodium | Face Centered Cubic (FCC) |
| Discovery of rhodium | By William Hyde Wollaston in 1804 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Rhodium element in Periodic table
The Rhodium element (Rh) has the atomic number 45 and is located in group 9 and period 5. Rhodium is a metal and it is classified as a transition element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about rhodium
Here are a few interesting facts about rhodium element.
- The name rhodium came from the Greek word “Rhodon” which means rose.
- Rhodium was discovered by William Hyde Wollaston in 1804.
- The concentration of rhodium in the earth’s crust is very less and it is 1 part per 200 million.
- Rhodium is obtained from the waste product of uranium fission.
- Around 87% of the total rhodium is used in catalytic converters.
Properties of rhodium
Here is a list of some physical properties and chemical properties of rhodium.
Physical properties of rhodium
- Rhodium is a solid metal that has a silvery white appearance.
- Rhodium is resistive to wear and it has a good hardness.
- The atomic mass of rhodium is 102.91 u and its density is 12.45 g/cm3.
- The melting point and boiling point of rhodium is 2237 K and 3968 K respectively.
- Rhodium has many isotopes, but the most abundant isotope is 103Ru.
- Rhodium has a FCC crystal structure.
Chemical properties of rhodium
- The electronic configuration of rhodium is [Kr] 4d8 5s1 and it is a transition metal as it has incomplete d-orbitals.
- Rhodium is very less reactive to acids.
- Rhodium is also called a noble metal as it does not react easily with oxygen.
- Rhodium is resistive to corrosion.
Uses of rhodium
Here are some uses of the rhodium element.
- Rhodium is used in catalytic converters (these are used in cleaning the vehicle emissions).
- Rhodium is also added to platinum and iridium. This makes the alloy heat resistant at higher temperatures.
- Rhodium is used as a coating metal on crucibles as well as coating on optical fibers.
- Rhodium is used as a catalyst in preparation of nitric acid and acetic acid.
External resources:
- Rhodium – Element information, properties and uses | Periodic Table. (n.d.). Rhodium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/45/rhodium
- Prohaska, T., et al. (2022, May 1). Standard atomic weights of the elements 2021 (IUPAC Technical Report). Pure and Applied Chemistry, 94(5), 573–600. https://doi.org/10.1515/pac-2019-0603
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Rhodium – Wikipedia. (2007, December 10). Rhodium – Wikipedia. https://en.wikipedia.org/wiki/Rhodium
- P. (n.d.). Rhodium | Rh (Element) – PubChem. Rhodium | Rh (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Rhodium
- Bondi, A. (1964, March). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), 441–451. https://doi.org/10.1021/j100785a001
- Holden, et al. (2018, December 1). IUPAC Periodic Table of the Elements and Isotopes (IPTEI) for the Education Community (IUPAC Technical Report). Pure and Applied Chemistry, 90(12), 1833–2092. https://doi.org/10.1515/pac-2015-0703
- Zhang, et al. (2011, January 11). Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. Journal of Chemical & Engineering Data, 56(2), 328–337. https://doi.org/10.1021/je1011086
- It’s Elemental – The Element Rhodium. (n.d.). It’s Elemental – the Element Rhodium. https://education.jlab.org/itselemental/ele045.html
- Rhodium (Rh) | AMERICAN ELEMENTS ® (n.d.). American Elements: The Materials Science Company. https://www.americanelements.com/rh.html
- Atomic Data for Rhodium (Rh). (n.d.). Atomic Data for Rhodium (Rh). https://physics.nist.gov/PhysRefData/Handbook/Tables/rhodiumtable1.htm
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – RHODIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – RHODIUM. https://pubsapp.acs.org/cen/80th/rhodium.html?
- Kaye, G W.C., & Laby, T H. Tables of physical and chemical constants. 15th Edition. United States.
- Sansonetti, J. E., & Martin, W. C. (2005, December). Handbook of Basic Atomic Spectroscopic Data. Journal of Physical and Chemical Reference Data, 34(4), 1559–2259. https://doi.org/10.1063/1.1800011
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.