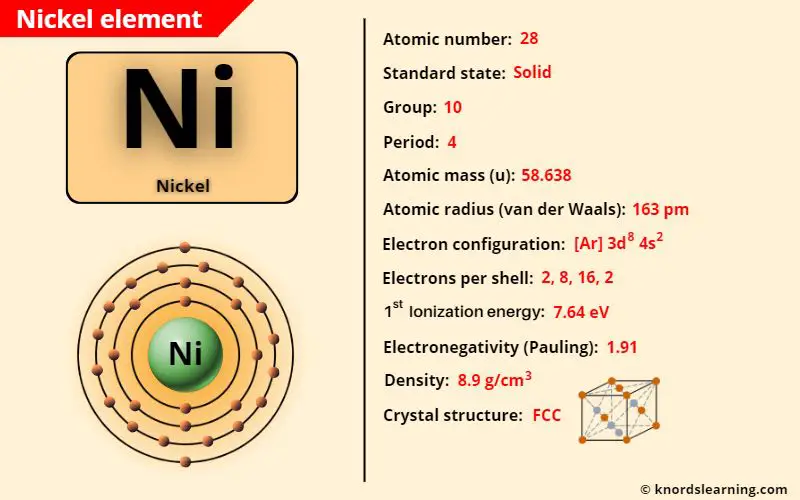
Nickel element (Ni) is in group 10 and period 4 of a periodic table. Nickel is in the d-block and it is classified as a transition element on the periodic table.
There is a lot more information related to nickel which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Nickel element (Information Table)
- Nickel element in Periodic table
- Facts about Nickel
- Properties of Nickel
- Uses of Nickel
Nickel Element (Information Table)
The important data related to nickel element is given in the table below.
| Appearance of nickel | Lustrous metallic appearance |
| Atomic number of nickel | 28 |
| Symbol of nickel | Ni |
| Atomic mass of nickel | 58.638 u |
| Protons, Neutrons & Electrons in nickel | Protons: 28, Neutrons: 31, Electrons: 28 |
| State of nickel (at STP) | Solid |
| Group number of nickel in periodic table | 10 |
| Period number of nickel in periodic table | 4 |
| Block of nickel in periodic table | d-block |
| Category of nickel | Transition metals |
| Bohr model or Electrons per shell or Electrons arrangement in nickel | 2, 8, 16, 2 |
| Electron configuration of nickel | [Ar] 3d8 4s2 |
| Orbital diagram of nickel | 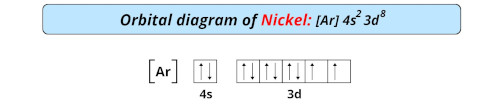 |
| Electronegativity of nickel (on pauling scale) | 1.91 |
| Atomic radius of nickel (van der Waals radius) | 163 picometers |
| Density of nickel | 8.908 g/cm3 |
| 1st ionization energy of nickel | 7.64 eV |
| Main isotopes of nickel | 58Ni (68% abundance) and 60Ni (26.2% abundance) |
| Melting point of nickel | 1728 K or 1455 °C or 2651 °F |
| Boiling point of nickel | 3003 K or 2730 °C or 4946 °F |
| Crystal structure of nickel | Face Centered Cubic (FCC) |
| Discovery of nickel | By Axel Fredrik Cronstedt in 1751 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Nickel element in Periodic table
The Nickel element (Ni) has the atomic number 9 and is located in group 17 and period 2. Nickel is a metal and it is classified as a transition element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about nickel
Here are a few interesting facts about nickel element.
- Nickel is the 5th most abundant element found from the earth. But if we talk about the proportion of nickel in the earth’s crust, then it is the 22nd most abundant element.
- If we talk about the inner core, then the proportion of nickel is the 2nd most abundant element. (Iron is the first).
- Nickel is ductile metal and 1kg of nickel can be drawn into 300km of long wire.
- In 1881, the first coin of pure nickel was made in Switzerland.
- Around 65% of the total nickel in the world is used in manufacturing of stainless steels.
- Nickel has magnetic properties which it can retain even at higher temperatures.
- Nickel is largely available from canada. It is believed that the large meteorite may have crashed near canada before 1000’s of years.
Properties of nickel
Here is a list of some physical properties and chemical properties of nickel.
Physical properties of nickel
- Nickel is a hard metal having a shiny silvery metallic appearance.
- The melting point and boiling point of nickel is 1728 K and 3003 K respectively.
- Out of all the isotopes of nickel, the most abundant isotopes are 58Ni and 60Ni.
- Nickel also shows magnetic properties.
- Nickel has an atomic mass of 58.638 u and its density is 8.908 g/cm3.
- Nickel is a good conductor of electricity and heat.
Chemical properties of nickel
- The electron configuration of nickel is [Ar] 3d8 4s2 and it has incomplete d-orbitals which is why it is classified as a transition metal on the periodic table.
- Nickel is less reactive metal and it shows less reactivity with other metals.
- Some glass shows a greenish tint, which indicates the presence of nickel in it.
- Nickel is resistive to corrosion.
Uses of nickel
Here are some uses of the nickel element.
- Nickel is used as a protective coating layer on other metals.
- The nickel metal is also used in making guitar strings.
- Nickel is used in making stainless steels as well as in some military applications.
- Nickel has a property to resist higher temperature and hence it is used to make alloys which can resist high temperatures.
- Nickel is also used in nickel-cadmium batteries, coin making etc because of its anti-corrosive nature.
- Nickel is used in electroplating of other metals (because it is difficult to oxidize).
External resources:
- Nickel Statistics and Information | U.S. Geological Survey. (n.d.). Nickel Statistics and Information | U.S. Geological Survey. https://www.usgs.gov/centers/national-minerals-information-center/nickel-statistics-and-information
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/28.shtml
- Nickel – Wikipedia. (2016, March 2). Nickel – Wikipedia. https://en.wikipedia.org/wiki/Nickel
- Nickel – Element information, properties and uses | Periodic Table. (n.d.). Nickel – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/28/nickel
- P. (n.d.). Nickel | Ni (Element) – PubChem. Nickel | Ni (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Nickel
- It’s Elemental – The Element Nickel. (n.d.). It’s Elemental – the Element Nickel. https://education.jlab.org/itselemental/ele028.html
- Atomic Weight of Nickel | Commission on Isotopic Abundances and Atomic Weights. (n.d.). Atomic Weight of Nickel | Commission on Isotopic Abundances and Atomic Weights. https://ciaaw.org/nickel.htm
- Atomic Data for Nickel (Ni). (n.d.). Atomic Data for Nickel (Ni). https://physics.nist.gov/PhysRefData/Handbook/Tables/nickeltable1.htm
- Nickel | Ni | ChemSpider. (n.d.). Nickel | Ni | ChemSpider. http://www.chemspider.com/Chemical-Structure.910.html?rid=fc14ffa3-f019-46b1-ad73-1e8c44839e3d&page_num=0
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – NICKEL. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – NICKEL. https://pubsapp.acs.org/cen/80th/nickel.html?
- Possolo, et al. (2018, January 4). Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report). Pure and Applied Chemistry, 90(2), 395–424. https://doi.org/10.1515/pac-2016-0402
- Emsley, J. (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. United Kingdom: OUP Oxford.
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Electronic structure of the elements. (2000, March). The European Physical Journal C, 15(1–4), 78–79. https://doi.org/10.1007/bf02683401
- James A. M. & Lord M. P. (1992). Macmillan’s chemical and physical data. Macmillan.
- Bedford, et al. (1996, April 1). Recommended values of temperature on the International Temperature Scale of 1990 for a selected set of secondary reference points. Metrologia, 33(2), 133–154. https://doi.org/10.1088/0026-1394/33/2/3
- Allred, A. (1961, June). Electronegativity values from thermochemical data. Journal of Inorganic and Nuclear Chemistry, 17(3–4), 215–221. https://doi.org/10.1016/0022-1902(61)80142-5
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

