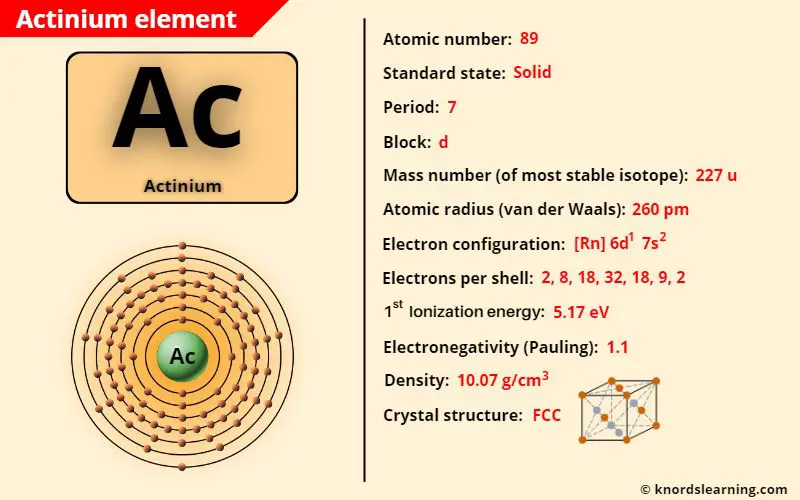
Actinium element (Ac) is in period 7 of a periodic table. Actinium is in the d-block and it is classified as an actinide on the periodic table.
There is a lot more information related to actinium which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Actinium element (Information Table)
- Actinium element in Periodic table
- Facts about Actinium
- Properties of Actinium
- Uses of Actinium
Actinium Element (Information Table)
The important data related to actinium element is given in the table below.
| Appearance of actinium | Silvery white |
| Atomic number of actinium | 89 |
| Symbol of actinium | Ac |
| Atomic mass of actinium (most stable isotope) | 227 u |
| Protons, Neutrons & Electrons in actinium | Protons: 89, Neutrons: 138, Electrons: 89 |
| State of actinium (at STP) | Solid |
| Period number of actinium in periodic table | 7 |
| Block of actinium in periodic table | d-block |
| Category of actinium | Actinides |
| Bohr model or Electrons per shell or Electrons arrangement in actinium | 2, 8, 18, 32, 18, 9, 2 |
| Electron configuration of actinium | [Rn] 6d1 7s2 |
| Orbital diagram of actinium | 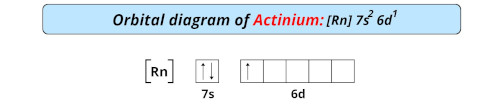 |
| Electronegativity of actinium (on pauling scale) | 1.1 |
| Atomic radius of actinium (van der Waals radius) | 260 picometers |
| Density of actinium | 10.07 g/cm3 |
| 1st ionization energy of actinium | 5.17 eV |
| Main isotope of actinium | 227Ac |
| Crystal structure of actinium | Face Centered Cubic (FCC) |
| Discovery of actinium | By Friedrich Oskar Giesel in 1902 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Actinium element in Periodic table
The Actinium element (Ac) has the atomic number 89 and is located in period 7. Actinium is a metal and it is classified as an actinide group element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about actinium
Here are a few interesting facts about the actinium element.
- Friedrich Oskar Giesel discovered actinium in 1902.
- Actinium was given its name from the Greek word “aktis”, which means beam.
- Actinium is 150 times more radioactive than radium.
- All the isotopes of actinium are radioactive in nature.
Properties of actinium
Here is a list of some physical properties and chemical properties of actinium.
Physical properties of actinium
- Actinium has a silvery white appearance.
- Actinium glows blue in color in the dark.
- Actinium has FCC crystal structure.
- The most stable isotope of actinium has the density 10.07 g/cm3 and its atomic mass is 227 amu.
Chemical properties of actinium
- When actinium reacts with the moist air, it forms a thin oxide layer (actinium oxide layer).
- The most common oxidation state of actinium is +3.
- Actinium is harmful for humans as it is radioactive in nature.
- Actinium damages the cells of the human body and it also causes bone cancer and other illnesses.
Uses of actinium
Actinium has no commercial uses because of its radioactive nature, but it is generally used for research work.
External resources:
- Actinium – Element information, properties and uses | Periodic Table. (n.d.). Actinium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/89/actinium
- Actinium – Wikipedia. (2011, June 2). Actinium – Wikipedia. https://en.wikipedia.org/wiki/Actinium
- P. (n.d.). Actinium | Ac (Element) – PubChem. Actinium | Ac (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Actinium
- It’s Elemental – The Element Actinium. (n.d.). It’s Elemental – the Element Actinium. https://education.jlab.org/itselemental/ele089.html
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – ACTINIUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – ACTINIUM. http://pubsapp.acs.org/cen/80th/actinium.html?
- Atomic Data for Actinium (Ac). (n.d.). Atomic Data for Actinium (Ac). https://physics.nist.gov/PhysRefData/Handbook/Tables/actiniumtable1.htm
- Possolo, et al. (2018, January 4). Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report). Pure and Applied Chemistry, 90(2), 395–424. https://doi.org/10.1515/pac-2016-0402
- Emsley, J. (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. United Kingdom: OUP Oxford.
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Electronic structure of the elements. (2000, March). The European Physical Journal C, 15(1–4), 78–79. https://doi.org/10.1007/bf02683401
- James A. M. & Lord M. P. (1992). Macmillan’s chemical and physical data. Macmillan.
- Bedford, et al. (1996, April 1). Recommended values of temperature on the International Temperature Scale of 1990 for a selected set of secondary reference points. Metrologia, 33(2), 133–154. https://doi.org/10.1088/0026-1394/33/2/3
- Allred, A. (1961, June). Electronegativity values from thermochemical data. Journal of Inorganic and Nuclear Chemistry, 17(3–4), 215–221. https://doi.org/10.1016/0022-1902(61)80142-5
- Actinium | Ac | ChemSpider. (n.d.). Actinium | Ac | ChemSpider. http://www.chemspider.com/Chemical-Structure.22404.html?rid=2b49da4d-087d-4376-b0f6-50ba25907a78&page_num=0
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

