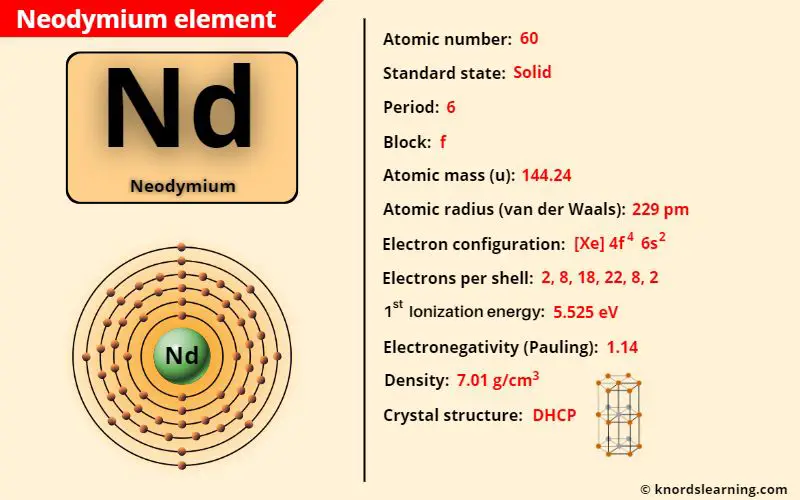
Neodymium element (Nd) is in period 6 of a periodic table. Neodymium is in the f-block and it is classified as a lanthanide on the periodic table.
There is a lot more information related to neodymium which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Neodymium element (Information Table)
- Neodymium element in Periodic table
- Facts about Neodymium
- Properties of Neodymium
- Uses of Neodymium
Neodymium Element (Information Table)
The important data related to neodymium element is given in the table below.
| Appearance of neodymium | Silvery white appearance |
| Atomic number of neodymium | 60 |
| Symbol of neodymium | Nd |
| Atomic mass of neodymium | 144.24 u |
| Protons, Neutrons & Electrons in neodymium | Protons: 60, Neutrons: 84, Electrons: 60 |
| State of neodymium (at STP) | Solid |
| Period number of neodymium in periodic table | 6 |
| Block of neodymium in periodic table | f-block |
| Category of neodymium | Inner transition metals |
| Bohr model or Electrons per shell or Electrons arrangement in neodymium | 2, 8, 18, 22, 8, 2 |
| Electron configuration of neodymium | [Xe] 4f4 6s2 |
| Orbital diagram of neodymium | 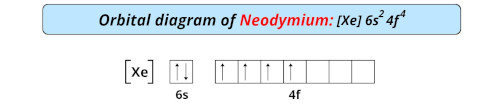 |
| Electronegativity of neodymium (on pauling scale) | 1.14 |
| Atomic radius of neodymium (van der Waals radius) | 229 picometers |
| Density of neodymium | 7.01 g/cm3 |
| 1st ionization energy of neodymium | 5.525 eV |
| Main isotope of neodymium | 142Nd |
| Melting point of neodymium | 1297 K or 1024 °C or 1875 °F |
| Boiling point of neodymium | 3347 K or 3074 °C or 5565 °F |
| Crystal structure of neodymium | Double Hexagonal Close Packing (DHCP) |
| Discovery of neodymium | By Carl Auer von Welsbach in 1885 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Neodymium element in Periodic table
The Neodymium element (Nd) has the atomic number 60 and is located in period 6. Neodymium is a metal and it is classified as a lanthanide group element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about neodymium
Here are a few interesting facts about neodymium element.
- Neodymium was given its name from the two Greek words “neos” and “dymium”.
- The concentration of neodymium in the earth’s crust is 33 ppm.
- Neodymium is the 2nd most abundant rare earth metal on the periodic table.
- Neodymium metal is not available from one place on the earth. But it is evenly spread on the earth.
- Carl Auer von Welsbach discovered neodymium in the year 1885.
Properties of neodymium
Here is a list of some physical properties and chemical properties of neodymium.
Physical properties of neodymium
- Neodymium has a silvery-gray metallic appearance.
- The crystal structure of neodymium is HCP.
- There are many isotopes of neodymium, and out of these isotopes the most abundant isotope is 142Nd.
- The density of neodymium is 7.01 g/cm3 and its atomic mass is 144.24 amu.
- The melting point and boiling point of neodymium is 1297 K and 3347 K respectively.
Chemical properties of neodymium
- Neodymium is reactive metal and hence it is always found in other compounds from the earth’s crust.
- The neodymium easily reacts with the oxygen of the atmosphere.
- Neodymium is kept under kerosene or a mineral oil to prevent its reaction with the atmospheric oxygen.
- Neodymium has an electronegativity of 1.14 on the pauling scale.
Uses of neodymium
Here are some uses of the neodymium element.
- Neodymium is used in the manufacturing of colored glasses.
- Nd:YAG lasers use a crystal of neodymium.
- The coloring agents used in enamels also have neodymium in it.
- Neodymium is also used in making strong magnets. The neodymium magnets are made by alloying neodymium, iron and boron.
External resources:
- Bizimis, M., & Scher, H. D. (2016, January 1). Neodymium Isotopes. Neodymium Isotopes | SpringerLink. https://doi.org/10.1007/978-3-319-39193-9_124-1
- Neodymium – Element information, properties and uses | Periodic Table. (n.d.). Neodymium – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/60/neodymium
- Possolo, et al. (2018, January 4). Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report). Pure and Applied Chemistry, 90(2), 395–424. https://doi.org/10.1515/pac-2016-0402
- Emsley, J. (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. United Kingdom: OUP Oxford.
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Electronic structure of the elements. (2000, March). The European Physical Journal C, 15(1–4), 78–79. https://doi.org/10.1007/bf02683401
- James A. M. & Lord M. P. (1992). Macmillan’s chemical and physical data. Macmillan.
- Bedford, et al. (1996, April 1). Recommended values of temperature on the International Temperature Scale of 1990 for a selected set of secondary reference points. Metrologia, 33(2), 133–154. https://doi.org/10.1088/0026-1394/33/2/3
- Allred, A. (1961, June). Electronegativity values from thermochemical data. Journal of Inorganic and Nuclear Chemistry, 17(3–4), 215–221. https://doi.org/10.1016/0022-1902(61)80142-5
- Neodymium – Wikipedia. (2012, August 16). Neodymium – Wikipedia. https://en.wikipedia.org/wiki/Neodymium
- Neodymium – American Chemical Society. (n.d.). American Chemical Society. https://www.acs.org/greenchemistry/research-innovation/endangered-elements/neodymium.html
- P. (n.d.). Neodymium | Nd (Element) – PubChem. Neodymium | Nd (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Neodymium
- It’s Elemental – The Element Neodymium. (n.d.). It’s Elemental – the Element Neodymium. https://education.jlab.org/itselemental/ele060.html
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/60.shtml
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.

