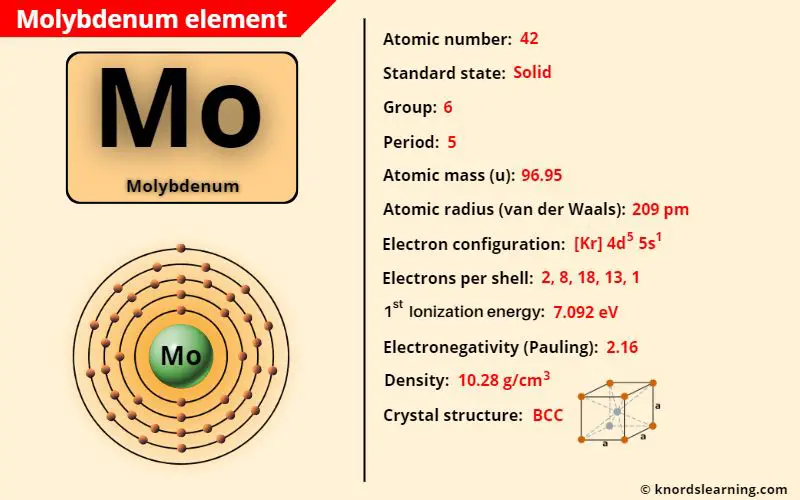
Molybdenum element (Mo) is in group 6 and period 5 of a periodic table. Molybdenum is in the d-block and it is classified as a transition element on the periodic table.
There is a lot more information related to transition which is mentioned in the Information Table given below.
So let’s dive right into it!
Table of contents
- Molybdenum element (Information Table)
- Molybdenum element in Periodic table
- Facts about Molybdenum
- Properties of Molybdenum
- Uses of Molybdenum
Molybdenum Element (Information Table)
The important data related to molybdenum element is given in the table below.
| Appearance of molybdenum | Gray metallic shiny appearance |
| Atomic number of molybdenum | 42 |
| Symbol of molybdenum | Mo |
| Atomic mass of molybdenum | 96.95 u |
| Protons, Neutrons & Electrons in molybdenum | Protons: 42, Neutrons: 54, Electrons: 42 |
| State of molybdenum (at STP) | Solid |
| Group number of molybdenum in periodic table | 6 |
| Period number of molybdenum in periodic table | 5 |
| Block of molybdenum in periodic table | d-block |
| Category of molybdenum | Transition metals |
| Bohr model or Electrons per shell or Electrons arrangement in molybdenum | 2, 8, 18, 13, 1 |
| Electron configuration of molybdenum | [Kr] 4d5 5s1 |
| Orbital diagram of molybdenum | 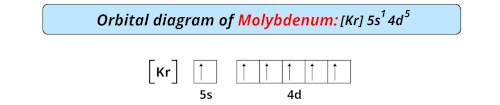 |
| Electronegativity of molybdenum (on pauling scale) | 2.16 |
| Atomic radius of molybdenum (van der Waals radius) | 209 picometers |
| Density of molybdenum | 10.28 g/cm3 |
| 1st ionization energy of molybdenum | 7.092 eV |
| Main isotopes of molybdenum | 95Mo, 96Mo, 98Mo |
| Melting point of molybdenum | 2896 K or 2623 °C or 4753 °F |
| Boiling point of molybdenum | 4912 K or 4639 °C or 8382 °F |
| Crystal structure of molybdenum | Body Centered Cubic (BCC) |
| Discovery of molybdenum | By Carl Wilhelm Scheele in 1778 |
Also see: Interactive Periodic Table (It has rotating bohr models as well as many other details of all the 118 elements in a single periodic table).
Molybdenum element in Periodic table
The Molybdenum element (Mo) has the atomic number 42 and is located in group 6 and period 5. Molybdenum is a metal and it is classified as a transition element.
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| *Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| **Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Click on above elements in the periodic table to see their information.
Facts about molybdenum
Here are a few interesting facts about molybdenum element.
- Molybdenum was given its name from the Greek word “molybdos”, which means lead.
- Molybdenum is the 54th most abundant element present in the earth’s crust.
- The concentration of molybdenum is 1.2 ppm (by weight) in the earth’s crust.
- Around 200,000 tons of molybdenum is produced annually in the world.
- Around 80% of molybdenum is used in steel manufacturing.
- Molybdenum ores are available in large quantities from China.
- The density of molybdenum is half the density of tungsten.
- Molybdenum is also present in plants as well as animals as a micronutrient.
Properties of molybdenum
Here is a list of some physical properties and chemical properties of molybdenum.
Physical properties of molybdenum
- Molybdenum has a gray metallic luster.
- There are many isotopes of molybdenum. Out of these, the common isotopes are 95Mo (15.8 %), 96Mo (16.6 %), and 98Mo (24.3 %).
- The atomic mass of molybdenum is 96.95 u and its density is 10.28 g/cm3.
- The melting point and boiling point of molybdenum is 2896 K and 4912 K respectively.
Chemical properties of molybdenum
- The electronic configuration of molybdenum is [Kr] 4d5 5s1 and it has incomplete d-orbitals.
- Molybdenum does not show any reaction with water at room temperature, but it reacts with it at higher temperature (above 600 °C) to form molybdenum trioxide.
- Molybdenum can form compounds with many other elements as it has many oxidation states.
Uses of molybdenum
Here are some uses of the molybdenum element.
- Molybdenum is used as an alloying element with other elements which gives improved properties like hardness, strength, etc.
- The powdered molybdenum is used in powder metallurgy.
- Molybdenum is used as a catalyst in petroleum industries.
- Molybdenum is also used in glass melting electrodes, because molybdenum can resist higher temperatures.
- Molybdenum is also used in making engine parts, drill bits, saw blades, etc.
External resources:
- Molybdenum – Wikipedia. (2007, December 10). Molybdenum – Wikipedia. https://en.wikipedia.org/wiki/Molybdenum
- Molybdenum – Element information, properties and uses | Periodic Table. (n.d.). Molybdenum – Element Information, Properties and Uses | Periodic Table. https://www.rsc.org/periodic-table/element/42/molybdenum
- P. (n.d.). Molybdenum | Mo (Element) – PubChem. Molybdenum | Mo (Element) – PubChem. https://pubchem.ncbi.nlm.nih.gov/element/Molybdenum
- Molybdenum | CCDC. (n.d.). Molybdenum | CCDC. https://www.ccdc.cam.ac.uk/elements/molybdenum/
- Molybdenum – Energy Education. (n.d.). Molybdenum – Energy Education. https://energyeducation.ca/encyclopedia/Molybdenum
- It’s Elemental – The Element Molybdenum. (n.d.). It’s Elemental – the Element Molybdenum. https://education.jlab.org/itselemental/ele042.html
- Periodic Table of Elements: Los Alamos National Laboratory. (n.d.). Periodic Table of Elements: Los Alamos National Laboratory. https://periodic.lanl.gov/42.shtml
- Atomic Weight of Molybdenum | Commission on Isotopic Abundances and Atomic Weights. (n.d.). Atomic Weight of Molybdenum | Commission on Isotopic Abundances and Atomic Weights. https://ciaaw.org/molybdenum.htm
- Atomic Data for Molybdenum (Mo). (n.d.). Atomic Data for Molybdenum (Mo). https://physics.nist.gov/PhysRefData/Handbook/Tables/molybdenumtable1.htm
- Molybdenum | Mo | ChemSpider. (n.d.). Molybdenum | Mo | ChemSpider. http://www.chemspider.com/Chemical-Structure.22374.html?rid=46f71f76-f19b-47e3-92b7-f1e1fd8d2463
- Molybdenum Statistics and Information | U.S. Geological Survey. (n.d.). Molybdenum Statistics and Information | U.S. Geological Survey. https://www.usgs.gov/centers/national-minerals-information-center/molybdenum-statistics-and-information
- C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – MOLYBDENUM. (n.d.). C&EN: IT’S ELEMENTAL: THE PERIODIC TABLE – MOLYBDENUM. https://pubsapp.acs.org/cen/80th/molybdenum.html?
- Haynes, W. M. (Ed.). (2014, June 4). CRC Handbook of Chemistry and Physics. https://doi.org/10.1201/b17118
- Emsley, J. (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. United Kingdom: OUP Oxford.
- Sansonetti, J. E., & Martin, W. C. (2005, December). Handbook of Basic Atomic Spectroscopic Data. Journal of Physical and Chemical Reference Data, 34(4), 1559–2259. https://doi.org/10.1063/1.1800011
- Bondi, A. (1964, March). van der Waals Volumes and Radii. The Journal of Physical Chemistry, 68(3), 441–451. https://doi.org/10.1021/j100785a001
- Kaye, G W.C., & Laby, T H. Tables of physical and chemical constants. 15th Edition. United States.
- James A. M. & Lord M. P. (1992). Macmillan’s chemical and physical data. Macmillan.
- Holden, et al. (2018, December 1). IUPAC Periodic Table of the Elements and Isotopes (IPTEI) for the Education Community (IUPAC Technical Report). Pure and Applied Chemistry, 90(12), 1833–2092. https://doi.org/10.1515/pac-2015-0703
- Allred, A. (1961, June). Electronegativity values from thermochemical data. Journal of Inorganic and Nuclear Chemistry, 17(3–4), 215–221. https://doi.org/10.1016/0022-1902(61)80142-5
- Zhang, Y., Evans, J. R. G., & Yang, S. (2011, January 11). Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. Journal of Chemical & Engineering Data, 56(2), 328–337. https://doi.org/10.1021/je1011086
- Possolo, A., van der Veen, A. M. H., Meija, J., & Hibbert, D. B. (2018, January 4). Interpreting and propagating the uncertainty of the standard atomic weights (IUPAC Technical Report). Pure and Applied Chemistry, 90(2), 395–424. https://doi.org/10.1515/pac-2016-0402
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations.
Read more about our Editorial process.